4.1 The monoamine hypothesis of mood disorders
In the 1950s it was noticed that around 20% of those patients prescribed the drug reserpine, used at the time to control high blood pressure, developed severe depression as a side effect.
It was subsequently discovered that reserpine depletes a group of neurotransmitters called monoamines, which include serotonin, noradrenalin and dopamine. Recall from earlier in this course that neurotransmitters are chemicals that neurons use to communicate with one another. Once neurotransmitter is released into a gap between neurons it must effectively bind to (join on to) an adjacent neuron in order to pass on its message. The point where it binds is known as a ‘receptor’. Every neuron therefore has a multitude of receptors for receiving neurotransmitter molecules. These receptors are like the glucocorticoid receptors that you saw in Activity 6.
Reserpine actually works inside neurons by preventing monoamines from being taken up into vesicles, leaving them vulnerable to being broken down inside neurons. Vesicles are small ‘bubbles’ inside neurons in which neurotransmitters are stored after they are made by the neuron and before they are released into synaptic gaps, the gaps between neurons – see Figure 9.)
What are the consequences if reserpine stops monoamine neurotransmitter from entering its vesicles?
There will be less of it available for release into the synaptic gap. So communication between neurons using this neurotransmitter will be hampered – cells are not able to signal to one another using this particular signal as effectively.
Around the same time it was noticed that tuberculosis patients, prescribed a different drug, isoniazid, sometimes experienced a lifting of pre-existing depression. Isoniazid inhibits (slows down or prevents the activity of) the substance monoamine oxidase (MAO). MAO is important because it breaks down monoamine neurotransmitters. As MAO destroys monoamines, inhibiting MAO would have the net effect of increasing the levels of monoamines available for neuron to neuron communication.
Such monoamine oxidase inhibitors (or MAOIs) became the first generation of antidepressants.
Subsequently it emerged that there was yet another way that available monoamine levels might increase: imipramine, another antidepressant, inhibited the reuptake of serotonin and noradrenalin that had been released into the synaptic gap (Figure 9). Reuptake is a clever process by which neurons try to reuse the neurotransmitter they have released. They simply take it back into themselves via special reuptake channels and try to package it neatly back into the vesicles (or storage bubbles) ready to be released the next time the neuron needs to signal to another neuron.
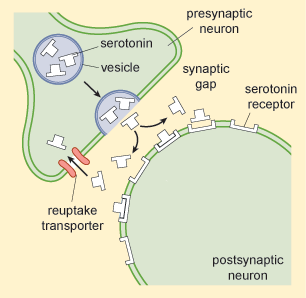
What effect would inhibition of reuptake have on neurotransmission involving monoamines such as serotonin and noradrenalin?
It would increase the amount of monoamines in the synaptic gap, so it would enhance neurotransmission as more would be available to bind to receptors enabling the process of neuron to neuron communication.
These findings about monoamines caused much excitement and led to the monoamine hypothesis of mood disorders. This postulated that monoamine levels have a primary role in causing depression, as lowering the levels of monoamines causes depression, while raising them lifts depression (see Hirschfeld (2000) for an overview). The mechanism postulated for this is shown in Figure 10.
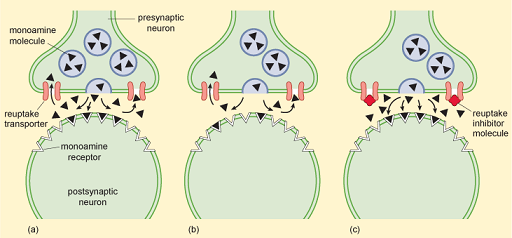
ADMs that inhibit the reuptake of specific monoamines such as serotonin (SSRIs, selective serotonin reuptake inhibitors), noradrenalin (noradrenergic reuptake inhibitors, NRIs), or combinations of monoamines such as serotonin and noradrenalin (serotonin noradrenergic reuptake inhibitors, SNRIs) are nowadays amongst the most prescribed drugs in Western societies, showing that the biomedical approach, and the monoamine hypothesis, still have a powerful influence on the treatment of depression. This is, in part at least, because many people see drugs as a ‘quick fix’ for depression and there is pressure to prescribe them.
Activity 7 Neurotransmitters and mood disorders
Name the main neurotransmitters implicated in the monoamine hypothesis of mood disorders. Are the levels of these neurotransmitters higher or lower in people who are depressed?
Answer
Serotonin and noradrenalin. Their levels are lower in those who are depressed.
