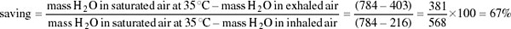3.4 Integration of anatomical features and biochemical and physiological strategies in endurers
The endurers, large animals with a relatively low surface area: volume ratio, have problems in losing heat from the body when exposed to high T a. Certain large lizard species behave like endurers, but they are evaders and evaporators too, a salutary reminder that we should not apply classification criteria too rigidly.
Dipsosaurus dorsalis, the desert iguana, lives in the Sonoran desert and is found most commonly in dry sandy areas where creosote bushes grow (Section 1.1).
Dipsosaurus is a plant eater, and it feeds on creosote bush leaves and flowers during the day, often being exposed to high solar radiation for up to 45 minutes.
The species was a puzzle to physiologists because it can attain a T b of up to 46°C, without any apparent ill-effects.
Activity 12
Why would a T b of 46°C appear to be incompatible with life in a complex vertebrate species such as Dipsosaurus?
Answer
The complex globular and fibrous proteins of most vertebrates are denatured at temperatures greater than 40°C.
Comparison of ATPase activity at different temperatures in a number of different lizard species showed that for a temperate species, Gerrhonotus multicarinatus, optimal temperature for ATPase activity is 30°C whereas for Dipsosaurus, it is about 41°C (Figure 36) and is still functional up to 43°C or 44°C.
Since Dipsosaurus has enzymes that are stable even at high T b it needs to expend less energy for thermoregulation, e.g. by shuttling, and can forage for longer during the day. By foraging during the day, Dipsosaurus avoids nocturnal predators such as foxes. Dipsosaurus relies on the moisture content of its diet for water. Reptiles do not have loops of Henle, but most are uricotelic, excreting uric acid and urate salts, not urea, which means that little water is excreted in these end products of protein metabolism.
Reptiles have some degree of physiological capacity to control their rate of change of body temperature. In Dipsosaurus dorsalis, radiant heating results in local cutaneous vasodilation, and local cooling results in cutaneous vasoconstriction. Panting and gaping have been observed in heat-stressed lizards such as Dipsosaurus dorsalis and Sauromalus obesus. When T b of Sauromalus exceeds 40°C, the mouth gapes, there is a five-fold increase in breathing rate, lung tidal volume decreases by two-thirds, and TEWL increases. Panting has significantly greater cooling effects on the brain than on the rest of the body, probably because of the proximity of the mouth to the carotid arteries in the neck that supply blood to the brain.
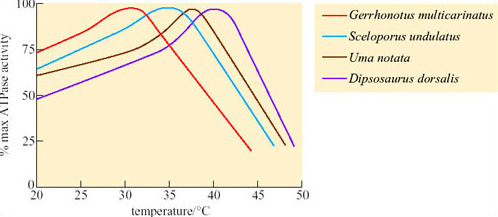
The best-known examples of desert endurers include the camel, the oryx and desert sheep; in fact most desert endurers are large mammals. Their relatively low surface area: volume ratio means that they have more difficulty than small animals in losing heat from the body at high T a. Mammalian and avian endurers are too large to shelter in burrows, and if no shade is available, they may be forced to remain exposed to solar radiation during the day. The hair of large desert mammals can play an important role in insulation, both from solar heat and nocturnal cold. Figure 37 compares the thermal properties of the coats of two breeds of heat-tolerant sheep with that of the camel.
For each example, the raised temperatures of the body core to 39°C suggest that some heat is being stored during the day. The long loose coat of the Awassi sheep is penetrated by solar energy which heats up the middle layers, making the skin quite hot. Merino sheep have a dense fleecy coat, and lose long-wave radiation from the hot tips of the hairs, maintaining a gradient of up to 43°C across 4–5 cm of fleece so the skin is protected from overheating. In short-coated camels, a dorsal ridge of long dense hair provides shading and insulation for the skin, while all of the coat, most of it short and smooth, reflects solar energy. Sweating keeps the camel's skin cool.
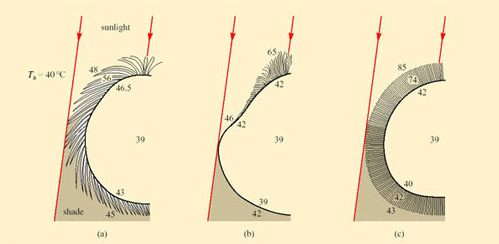
Sweating, an extreme form of CEWL in mammals, is important for cooling in many species. Sweating is the secretion of water plus some salts from special sweat glands in the skin, which occurs as a response to an increase in T b. The glands are of two types. Atrichial (without hair) glands are found in primates and also on the pads of cats and dogs. They develop from the epidermis independently of the hairs and open on the free surface of the skin. Atrichial sweat glands are at their densest on human palms and soles, and elsewhere on the body they are at a density of 100–300 cm−2 (Figure 38a). Epitrichial (around hair) sweat glands develop only in association with hair follicles (Figure 38b). They are found in many mammalian species including cattle, sheep, horses and camels.
Epitrichial sweat glands play an important role in thermoregulation in these species. In cattle there are about 1800 cm−2, and in sheep, about 300 cm−2. Sweat is an ultrafiltrate of plasma, containing sodium chloride and other salts, lactic acid and urea. As the sweat glands absorb much of the electrolytes, sweat is hypotonic to plasma; the salt content of sweat falls with acclimatisation. Evaporation of sweat, promoted by input of heat energy from the skin, cools the body. One problem for thermoregulation by sweating in furry mammals is that water evaporating from a fur coat takes a significant proportion of its heat from the air rather than from the skin, and is therefore less effective in cooling the body.
Sweating has a physiological cost in that it involves loss of salts and organic molecules as well as water from the body. Initially fluid lost from the plasma is replaced from various ‘non-essential’ reserves, such as the digestive glands and the gut, but eventually the osmotic pressure of the blood increases. The change is detected by special neurons, osmoreceptors, in the hypothalamus. In turn the osmoreceptors stimulate release of ADH from neurosecretory neurons in the hypothalamus. Secreted ADH enters the capillary network supplying the posterior pituitary, and from there the hormone is secreted into the bloodstream. ADH binds to receptors in cells lining the collecting ducts of the kidney and promotes resorption of water back into the bloodstream (Figure 39). Later on some fluid is withdrawn from the intracellular compartments of the body and there is an inevitable reduction in plasma volume if the lost water is not replaced by drinking.
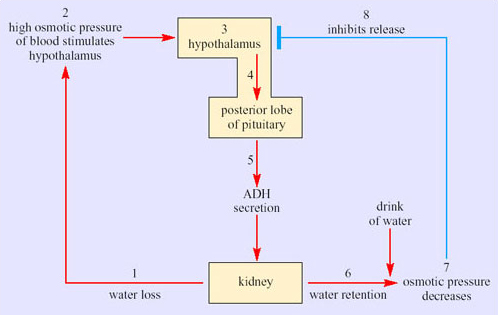
It is worthwhile working through the series of events in Figure 39. Stage 1 represents a situation where water lost via urine is not replaced by drinking water. The osmolarity of the blood increases, which raises the osmotic pressure of the blood. At stage 2, osmoreceptors in the hypothalamus detect the increased osmotic pressure of the blood. Hypothalamic neurosecretory neurons (stage 3) respond by transferring ADH along axons into the blood circulation of the posterior pituitary (stage 4). ADH secreted into the bloodstream (stage 5) reaches target cells, epithelial cells in the collecting ducts of kidney. The response to ADH is an increased permeability of the basal membranes of epithelial cells in the collecting ducts, which promotes resorption of water. The kidney thereby produces more concentrated urine and water is retained (stage 6). Say the individual now has a drink of water. Absorption of the water into the bloodstream decreases the osmolarity of the blood so the osmotic pressure decreases (stage 7). In stage 8, the hypothalamic receptors respond to the decline in osmotic pressure by inhibiting the release of ADH.
Activity 13
Is the control of ADH secretion an example of negative or positive feedback?
Answer
It is negative feedback because an increase in the secretion of ADH leads to a change in osmotic pressure, which in turn decreases ADH secretion.
The hormonal control of physiological variables by means of negative feedback loops maintains a constant level of body fluid volume, an important element of homeostasis, the maintenance of a constant internal environment.
Unfortunately in humans, the plasma contributes a disproportionate share of the overall fluid lost. A level of dehydration equivalent to a 4 per cent loss of body weight is accompanied by a reduction in plasma volume of about 10 per cent. Since there is no corresponding fall in blood cells or plasma proteins, the viscosity of the blood rises just at the time when the circulatory system, which bears the main burden of maintaining the body's heat balance, is already under strain as a result of the maximal cutaneous vasodilation that transports heat to the surface and plasma to the sweat glands. The demand on the heart is therefore great as it tries to maintain the blood pressure and peripheral circulation. Hypotension (low blood pressure) and fainting may follow. If correct action is not taken quickly, the result is a rapid rise in body temperature and death through hyperthermia.
The amount of water lost during sweating in humans can be considerable. A man marching in the desert may take on a solar and terrestrial radiation load of 2000 kJ h−1 even allowing for the reflectance of his skin and clothing. On the basis of a latent heat of vaporisation of 2.4 kJ g−1, about 800 cm3 of sweat would be needed per hour to dissipate this heat load even on the assumption that all the heat of vaporisation is drawn from the body itself. This calculation does not take into account any direct heat loading from air hotter than the body, or the substantial metabolic generation of heat. Therefore, figures of 2 or 3 litres of sweat per hour are feasible. An adult man carrying out moderate work in hot dry conditions may secrete 0.5 litres of sweat per hour and when under high heat stress, up to 3 litres per hour. The efficiency of the human sweating mechanism depends critically on the ability to drink. However, under prolonged heat stress, even if water is available, the limited capacity of humans for physically drinking water leads to a ‘voluntary’ dehydration of 2–4 per cent of the body mass, producing a chronic condition of decreased plasma volume and a raised blood osmotic pressure, coupled with a fall in urine output of up to 80 per cent.
The camel handles its water balance problems more effectively than humans. Schmidt-Nielsen et al. (1967) showed that in a 290 kg camel, a 17 per cent weight loss due to dehydration was accompanied by only an 8.8 per cent reduction in plasma volume and a 38 per cent fluid loss from the gut. A camel has up to 75 litres of fluid in its rumen (85 per cent of it is water) and another 8 litres in the intestine. The 38 per cent of water loss in the gut is therefore about 30 litres, which minimises strain on the blood circulation during dehydration. Overall, camel tissues seem to be more resistant to high osmotic pressures than those of many animals. The camel can cope with up to 30 per cent water loss. When provided with water after such a high level of dehydration, the camel can drink rapidly taking in up to 200 litres of water in just a few minutes. Much of the water taken in is stored temporarily in the gut, preventing excessive dilution of the blood which in itself would be harmful.
Schmidt-Nielsen et al.'s research added detail to the common perceptions that camels store free water in the rumen and utilise water derived from metabolism of the lipids released from the adipose tissue that forms the fatty hump. Although camels have a great deal of water in the rumen and intestine, it is proportionately no more than is present in other ruminants. It is true that since the oxidation of 1 g of fat yields 1.07 g water, a 40 kg hump could yield 43 litres of water that can be drawn on during a long journey. Schmidt-Nielsen pointed out that as this mechanism requires oxygen, which can only be obtained by ventilating the lungs, there must be a net loss of water from the respiratory tract when the camel breathes dry air. Whether the fat reserves make a positive or negative contribution to the animal's overall water balance depends on conditions prevailing in the upper respiratory tract.
Research by Schmidt-Nielsen and his colleagues investigated this question. Working on feral camels under natural conditions in central Australia, the researchers measured body temperatures, oxygen consumption and respiratory frequency and volume, as well as the temperature and relative humidity of the inspired and expired air by means of sensors inserted loosely into a nostril. The results showed that during the daytime, severely dehydrated camels exhaled air that was at or near the core temperature and fully saturated with water vapour. At night, however, there was a marked difference, the exhaled air being at or near the ambient temperature with a relative humidity of about 75 per cent.
We can best gain an impression of the camel's water balance by taking an example which uses hypothetical but realistic figures. Consider a camel resting at night in air at 28°C and 40 per cent r.h. Every 2 minutes it metabolises 1 litre of oxygen, extracted from 20 litres of inhaled air. This volume of air, under these conditions, contains 216 mg water. This same volume exhaled at core temperature (35°C) and fully saturated, contains 784 mg water, an overall loss to the camel of 568 mg. If 20 litres of saturated air were exhaled at ambient temperature (28°C, water content 538 mg), the loss would be reduced to 322 mg, while 20 litres of exhaled air at 28°C and only 75 per cent r.h. (the humidity measured by Schmidt-Nielsen) would have a water content of 403 mg, further reducing water loss to 187 mg. The maximum saving of water could then be calculated as follows:
Although this mechanism only seems to operate at night, it clearly makes an important contribution to water conservation in the dehydrated camel and probably ensures that there is a gain of water from the oxidation of fat.
Camels also conserve water by reducing urine flow, and can do so to a far greater extent than humans. The camel kidney can produce urine twice as concentrated as that of humans (see Table 3 in Section 3.2), and can reduce the amount of urea it excretes. According to Schmidt-Nielsen, urea in the blood may be secreted into the rumen, where bacteria incorporate the nitrogen into amino acids and then into protein. This protein is later digested and absorbed, and some of it is deaminated in the liver, releasing urea back into the blood again. This cycle may help to retain nitrogen, and hence the water that would have been needed to excrete it as urea, within the body during periods of water shortage.
Relaxed homeothermy is important for water conservation in the camel, which uses the capacity of its immense bulk (up to 500 kg) to store heat. If water is short in the summer, the camel may maintain a normal core temperature throughout the night and then at around 06.00 h allow its body temperature to fall to about 34°C. Throughout the day, its temperature rises due to muscular work, solar radiation or both, but the camel does not begin thermoregulating by sweating until the rectal temperature reaches 40°C. Thus the camel absorbs sufficient heat to raise its body temperature over a range of 6°C, compared with only 2°C in humans. By this means the camel may save about 5 litres of sweat in a day, which is significant, considering that a 500 kg animal resting in the sun loses a total of about 10 litres of water per day by sweating, breathing and excreting. There is no evidence that the camel's lethal body temperature is significantly different from that of humans, but it certainly is more tolerant of changes in body temperature.
Overall the anatomy, physiology and biochemistry of the camel are geared towards conservation of water. Camels survive without drinking for up to 6 weeks during colder periods, but in summer they must drink every 4 days at least. In contrast, Arabian oryx (Oryx leucoryx; see Figure 5 in Section 1.1) live in the Arabian desert, with no access to free-standing water. A group of wild oryx living in Mahazat as-Sayd, a nature reserve in central Saudi Arabia, have been studied extensively by Williams et al. (2001). Mahazat as-Sayd has no standing water apart from puddles after infrequent rain showers. Plants, mostly grasses and small trees (Acacia tortillas and Maerua crassifolia), cover only about 21 per cent of the reserve; the rest is sand. Summers are hot, with maximum and minimum temperatures of 41.5°C and 24.5°C respectively, and mild winters, with maximum and minimum temperatures of 23.4°C and 10.6°C. The annual rainfall is low, and falls mainly in winter: typical annual rainfall values were 129.6 mm in 1996, 84.3 mm in 1997. Tracking and observations of oryx showed that they feed on three species of grasses (Table 6).
| Summer 1998: % water content of grasses | Spring 1999: % water content of grasses | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | June | July | August | Mean | February | March | April | Mean |
| Stipagrostis sp. | 4.6 ± 2.5 | 12.7 ± 10.5 | 4.4 ± 0.4 | 7.2 | 38.6 ± 8.1 | 45.7 ± 4.9 | 34.5 ± 8.5 | 39.6 |
| Panicum turgidum | 45.4 ± 3.4 | 44.5 ± 5.3 | 41.4 ± 5.5 | 43.8 | 47.1 ± 4.9 | 51.9 ± 5.5 | 49.5 ± 4.4 | 49.5 |
| Lasiurus scindicus | 35.7 ± 5.3 | 30.4 ± 5.6 | 27.0 ± 14.5 | 31.0 | 40.5 ± 8.2 | 55.6 ± 9.5 | 52.5 ± 5.9 | 49.5 |
BMR and TEWL at 30°C were measured in oryx resting in specially constructed metabolic chambers (Table 7a). FMRs and field water influx rates (FWIR) in wild Arabian oryx living at Mahazat as-Sayd were measured by use of the doubly-labelled water technique (Table 7b). FWIR give a measure of the water intake of the animal. In the summer, six oryx returning in the morning from night foraging to rest in shade were darted with anaesthetic, then weighed and injected with doubly-labelled water, 2H218O. Blood samples were taken on the injection day and at a mean of 8 days post-injection. The results of assays of 2H and 18O content of blood and fecal samples were used to calculate rates of water intake and field metabolic rates for each animal (Table 7). The mean body mass of 12 oryx studied in 1998–1999 was 85 kg. In summer 1998, when grasses were parched, field metabolic rates of free-ranging oryx were 11 076 ± 3070 kJ day−1 in contrast to 22 081 ± 3646 kJ day−1 in spring 1999, after rains. The difference between metabolic rate in summer and spring was significant (PT a.
| Number and sex of animals | Mean body mass/kg | BMR/kJ day−1 | TEWL/g H2O day−1 | FMR/kJ day−1 | FWIR/cm3 day−1 |
|---|---|---|---|---|---|
| (a) 6 females | 89.2 | 9160 ± 732 | 898 ± 126 | — | — |
| (a) 6 males | 79.0 | 8674 ± 565 | 829 ± 181 | — | — |
| P/data pair | P> 0.06 | P | P | ||
| (b) 3 males + 3 females | 81.5 | — | — | 11076 ± 3070 | 1310 ± 1019 |
| (in summer 1998) | |||||
| (b) 5 males + 1 female | 89.0 | — | — | 22081 ± 3646 | 3438 ± 1006 |
| (in spring 1999) | |||||
| P/data pair | P> 0.5 | P | P |
Activity 14
Compare the mean water intake rates in the oryx in spring and summer, and state whether you think the differences you identify are statistically significant.
Answer
In spring, mean water intake is much greater, 3438 ± 1006 cm3 day−1 compared with 1310 ± 1019 cm3 day−1 in the summer. The difference in water intake certainly looks statistically significant (in fact, it is, P
Activity 15
Looking at the data in Tables 6 and 7, can you suggest a reason for the difference in water intake rates for oryx in spring and summer?
Answer
As no free-standing water is available to the oryx they must have obtained all or most of their water from their diet. Water content of grasses eaten by oryx varied significantly between summer and spring (see Table 6). Water content of Stipagrostis was just 7.2 per cent in summer 1998, but in spring 1999 rose to 39.6 per cent. Water content of L. scindicus rose from 31 per cent in summer 1998 to 49.5 per cent in spring 1999. Water content of P. turgidum was similar in spring and summer. As oryx only obtain water from their diet, presumably the lower water content of two of the food species determines a lower water intake in the summer.
Herbivores usually have a high water intake rate because of the high water content of plants. Williams et al. were surprised by the low values for water intake in oryx even during periods when the plants contain high percentages of water. Survival of a large herbivore on such low water intake suggests effective behavioural and physiological mechanisms that reduce water loss are in place. The simple strategy of resting in shade during the day and foraging at night (Section 2.5) plays an important role in reducing water loss.
Oryx, like camels, allow their body temperature to increase during the heat of the day thereby reducing the need for cooling by evaporative water loss. The temperature gradient in the nasal passages of both oryx and camel is used to protect the brain from overheating during periods of hyperthermia. The anatomy of the blood vessels in the head permits this protective mechanism. In both mammals and birds, blood returning from the nasal regions (and much of the brain) drains into a capacious collecting vessel, the cavernous sinus, from which it flows in the neck veins back to the heart. In many species including dogs, sheep, pigeons, and gazelles, a network of small arterial vessels, the rete mirabile (‘wonderful net’) passes through the cavernous sinus, branching from the two main arteries supplying the brain, the two carotid arteries. In species where nasal heat exchange is unimportant, e.g. monkey (Figure 40a) there is no rete mirabile and the carotid artery passes intact through the cavernous sinus providing little opportunity for exchange of heat. In the oryx, where the rete is present (Figure 40b), its large surface area permits exchange of heat between the warm arterial blood and the cooler blood in the cavernous sinus.
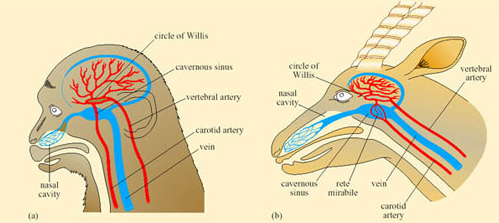
So blood passing out of the arterial rete and entering the circle of Willis, from which the brain receives its blood supply, is at a lower temperature than it was in the neck arteries themselves. As a result, the brain can be maintained at a lower temperature than the trunk and its essential function is maintained during hyperthermia in the rest of the body. The oryx and Thompson's gazelle can maintain a brain–body temperature difference of up to 3°C during sustained hyperthermia. This facility must be of enormous advantage to desert species when heat storage is used to minimise the physiological consequences of water shortage.
Large birds such as the ostrich allow their body temperature to rise when short of water and exposed to high T a. In fact both desert and non-desert species of bird can tolerate T b of 32–44°C, and reduce evaporative cooling when water is in short supply. The ostrich, like other ratites, has a long nose with complex turbinates and the nostrils are relatively far forward on the beak compared with their position in other birds. Expired air is cooled as it passes through the turbinates.
Ostriches can exhale unsaturated air. At T a = 36°C, exhaled air is at 85 per cent r.h. and by this means about 35 per cent of the water evaporated into the air during inspiration is saved. Thus the ostrich saves about 500 g water per day. However, you may be thinking that while water is conserved, the body does not lose any heat overall. The cooling effect in the turbinates does achieve brain cooling, with a 1.5°C difference measured between brain and body temperature. When at serious risk of overheating the ostrich supplements behavioural cooling strategies with panting by up to 40 times per minute.
Our study of a few representative examples of desert vertebrates has demonstrated a range of integrated anatomical, biochemical, and physiological adaptations and responses that enable the animals to cope with problems of high T a and water shortage. Yet there is nothing spectacular about desert animals; no unique and ‘amazing’ feature has been identified for desert vertebrates. Desert vertebrates rely heavily on behaviour, and we should ensure that we integrate behaviour with the overall package of biochemical and physiological adaptations for each species. Behavioural responses to environmental stresses reduce the need for physiological responses, which may be costly in terms of evaporative water loss or use of energy in an arid environment that has low productivity.