Effects of pollutants on the aquatic environment
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Tuesday, 16 April 2024, 1:43 PM
Effects of pollutants on the aquatic environment
Introduction
We can all relate to water. We know we need it to survive – indeed, the early great civilisations of Egypt and Mesopotamia were centred on river valleys where there was a plentiful supply of fresh, clean water.
When we take water into our bodies, it is used in several ways. For example:
- for cooling – it helps keep our bodies at around 37 °C
- as a waste disposal medium
- as a conductor for nerve impulses
- as a component in the digestion of food
- as a solvent in which vital chemical reactions take place.
You can see from the above that even if you didn’t move an inch, your body would still need water to keep you alive.
Water is a fascinating subject, encompassing chemistry, biology and physics. Apart from keeping us alive, water is used extensively in industrial processes, for recreation and for transport. It is something we can’t do without.
The water we use for domestic purposes ought to be free from contaminants, yet water pollution is a major problem in many countries. According to the World Health Organization (WHO, 2002), about 1.7 million people die each year due to unsafe water, sanitation and hygiene. In this text we consider in outline the major sources of pollution and the effect that pollutants have on the aquatic environment.
The activities located throughout the text will help you to review and remember what you have read.
This OpenLearn course is an adapted extract from the Open University course T868 Environmental monitoring and protection.
Learning outcomes
After studying this course, you should be able to:
list the major sources of water pollutants;
describe the effects on water due to organic materials, plant nutrients and toxic, physical and biological pollutants;
list the main diseases caused by microorganisms that can be carried by water.
1 The hydrological cycle
The hydrological cycle – the continuous cycling of water between land, open water surfaces and the sea, either directly or indirectly – is a complex process that has been known about for a long time (Figure 1). Probably the oldest reference to the hydrological cycle is found in the Chandogya, one of the principal Upanishads, which says ‘rivers … lead from sea to sea’. It reveals that as early as 1000 BCE, attempts were being made to interpret and explain recurrent phenomena on the basis of direct experience.

Figure 1 shows a group of cavemen with spears sitting on the ground in front of a large boulder. The boulder has a drawing of the sun, mountains, clouds, fields and animals. In front of the boulder, a caveman, using a spear as a pointer, is explaining the hydrological cycle to those gathered.
The identifiable mechanisms of the cycle are complicated not only by the characteristics of air–water–land interfaces across which the cycle operates, but also by climatic factors that vary in both time and space. The various operations and mechanisms within the cycle are illustrated in Figure 2.
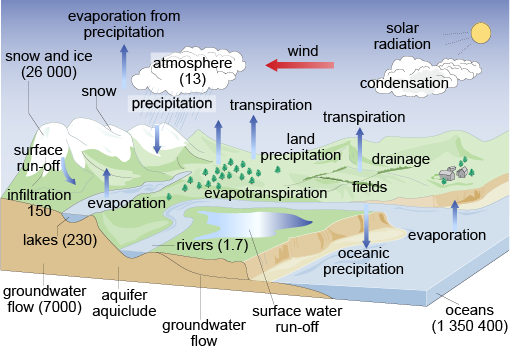
Figure 2 is a drawing showing clouds, the wind, mountains, snow and ice, a river, fields, a forest, houses, a lake, groundwater flow, and the sea. There are volumes in tera cubic metres (1012 m3) against certain items:
atmosphere – 13
snow and ice – 26 000
infiltration – 150
lakes – 230
rivers – 1.7
groundwater flow – 7000
oceans – 1 350 400.
There are upward-pointing arrows indicating loss to atmosphere: evaporation from the lake; transpiration from the forest and fields; evaporation from precipitation (rain); and evaporation from the sea.
There are downward-pointing arrows for precipitation, surface run-off from the mountains and oceanic precipitation.
2 Sources of pollution
To some extent a river is a self-renewing resource. If polluting discharges to a river are intermittent, the river is often able to return to a clean and unpolluted condition as the pollutants are flushed out and carried down to the sea. In addition, because of the organisms present (e.g. bacteria capable of breaking down organic matter), river water has some capacity for self-purification – unless too many of these organisms are killed off too quickly.
Some pollutants are objectionable because they overload the self-purification processes of the river. As rivers are often the raw water sources for potable supplies, this can have dire consequences.
An example of such pollution is the discharge of domestic sewage effluent to rivers. In small quantities it does no serious harm and may indeed be beneficial, providing a source of organic carbon that provides nutrients to the animals in the river. But if inadequately treated or in excessive quantities, sewage effluent can seriously damage the plant and animal life of a river by reducing the oxygen content of the water. In extreme cases, where the oxygen content is reduced to zero (or nearly so), the river will support very little life, and will become foul smelling and grossly offensive. A river in such a state is obviously not desirable as a water source for potable supply.
Some industrial effluents discharged in large quantities can be similarly harmful. For example, effluents from the food industry are not particularly toxic, but because of their organic content and large volume, they can exert a considerable oxygen demand on the environment in the region of the discharges.
The two pollution sources described above are classified as point sources, as the pollutants are generally collected by a network of pipes or channels and conveyed to a single point of discharge. Non-point or diffuse sources are characterised by multiple discharge sources that cannot be pinpointed. An example of a diffuse source is run-off from fields and roads.
Point sources are easily controlled, but diffuse sources are virtually impossible to collect and control. The latter pose great challenges in efforts to upgrade the quality of rivers.

Figure 3 is a photograph of a lake surrounded by trees. The surface of the water is covered in a bright green layer.
Lakes are much more vulnerable than rivers to pollution. Once a pollutant enters a lake it will stay for a long time. The flushing effect that characterises rivers is much less evident in lakes, and the dilution factor is much less than is available in the sea. Only the self-purifying ability of the water will abate the pollution in the long term. Lakes are thus particularly prone to eutrophication (Figure 3).
Since river pollutants can be controlled more easily at source, it is useful to know where they originate. A list of specific sources would be very long, but the following categories can be identified:
- discharges from sewage works, which often contain some industrial wastes
- discharges from manufacturing and industrial plants, including mines
- discharges from animal rearing, fish farming and agriculture
- seepage from domestic and industrial landfill sites
- urban surface water run-off.
Problems occur when the natural characteristics of a river are altered by pollutant discharges. We now consider the effects of the following categories of pollutants:
- organic materials
- plant nutrients
- toxic pollutants
- physical pollutants
- biological pollutants.
Please be aware that this categorisation is not absolute. For instance, toxic pollutants may well be organic, too.
3 Organic materials
Organic substances constitute the major freshwater pollutants, coming from domestic sewage discharges (even after treatment) and from certain industries such as food processing. This section will deal with the biodegradable forms, but there are also inert (non-biodegradable) toxic forms. Organic substances can be natural (in which case they are normally biodegradable) or synthetic (in which case they can often be degraded by microorganisms that have adapted to utilising them).
The major polluting effect of biodegradable organic materials is the reduction in oxygen concentration in the water. Bacteria and other organisms (decomposers) break these materials down into simpler organic or inorganic substances. They use up oxygen in the process, and as their population increases there is an extra demand for dissolved oxygen.
When a potentially polluting effluent is released into a stream, there follows a sequence of events in time and distance. This sequence leads to different environmental consequences and different aquatic communities compared with those immediately upstream and the successive reaches downstream. After a certain distance, natural biodegradative processes will break down the pollutants, often returning the river to something like its original condition.
Three stages of organic pollution can be defined.
- When the load is small, there will be little change in the species of plants and animals present in the water and little variation in the natural cycles. Initially, the dissolved oxygen will be near to saturation level. Any organic pollution apparent at the point of discharge will disappear within a short distance downstream as it is removed by the natural processes of self-purification. It could be said that, in some instances, mild organic pollution is beneficial to the river, since it increases the nutrient supply for microorganisms present in the natural state. This minimal pollution can benefit the whole aquatic ecosystem.
- If the load increases, the dissolved oxygen level will drop significantly and the river will be polluted for a considerable distance from the point of discharge. Some species of animals and plants will flourish at the expense of others. In the absence of further pollution, the river will probably recover downstream, but if the area around the discharge remains polluted then this can act as a barrier to the passage of migratory fish (among other disadvantages).
- If the polluting load is increased still further, the natural ecosystem will be grossly distorted and its effectiveness in coping with the pollutant load greatly reduced. The level of dissolved oxygen will be very low or fall to zero. Often the only organisms to flourish will be sewage fungus, certain worms and fly larvae. (These organisms give a low Biological Monitoring Working Party (BMWP) score, indicating the polluted nature of the river.) Also, anaerobic bacteria may thrive and give a foul smell to the water by metabolising organic substances and producing methane, hydrogen sulfide and ammonia. Few algae are able to thrive under severe organic pollution, so reoxygenation by photosynthesis will be hindered. The river will now remain polluted for a much greater distance downstream.
The sequence of events following significant pollution of a waterway by organic material is shown in Figure 4.
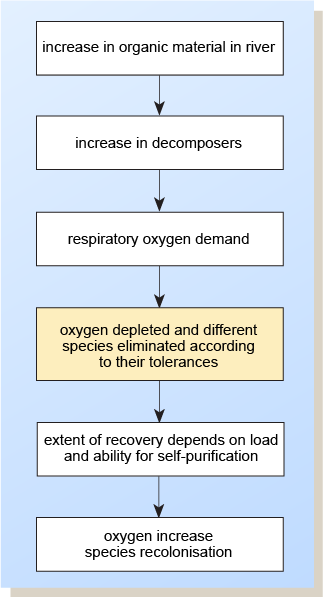
Figure 4 is a series of six rectangular boxes, one above the other, and connected by arrows pointing downwards. Starting from the top, they have the following wording: increase in organic material in river; increase in decomposers; respiratory oxygen demand; oxygen depleted and different species eliminated according to their tolerances; extent of recovery depends on load and ability for self-purification; oxygen increase, species recolonisation.
Inorganic materials can also cause deoxygenation, e.g. when ferrous iron from mine drainage water enters a river (Stumm and Lee, 1961). In the reduced ferrous (Fe(II)) state the iron is in solution, but on meeting the oxygen in the river it is oxidised to red insoluble ferric (Fe(III)) iron, a process that reduces the concentration of dissolved oxygen in the river water. The oxidised iron is now in suspension so that, as well as reducing the oxygen content, it reduces light penetration. It finally settles out slowly downstream of the discharge point, giving rise to all the problems associated with suspended solids. This type of problem is usually associated with coal-mining effluents.
Activity 1
a.
a. Depletion of oxygen occurs because of an increase in the activities of primary producers in both cases.
b.
b. In both cases it is the presence of plant nutrients (nitrates and phosphates) that causes the death of green plants and depletion of oxygen.
c.
c. The difference in the proportion of producers and consumers between organically polluted waters and eutrophic waters is negligible.
d.
d. A reduction of dissolved oxygen in both cases causes the depletion of species.
e.
e. Once the dissolved oxygen content is decreased, only the removal of the offending pollutant can allow an increase in species.
The correct answers are d and e.
Answer
Statements (d) and (e) are true.
Statement (a) is false: depletion of oxygen occurs in both cases due to the activities of decomposers. An increase in primary producers (plants) would increase oxygen levels.
Statement (b) is false: only in eutrophication are plant nutrients present, and these will usually encourage plant growth.
Statement (c) is false: in organically polluted waters the producers are only a small proportion of the ecology, making up less than 25% of the population, whilst in eutrophic waters they constitute more than 75%. The proportion of consumers, on the other hand, is similar in both situations (though slightly greater in organically polluted waters).
4 Plant nutrients
Certain inorganic substances are essential for normal plant metabolism but they can reach such levels as to be considered pollutants.
Eutrophication is the increase with time of plant nutrients and biota in a watercourse. Farming contributes 50–60% of nitrates and 20–30% of phosphorus getting into UK waters (GOV.UK, n.d.), with most of the remainder coming from treated sewage effluent. Pollutants from these sources can greatly accelerate the natural process by increasing the inputs of nutrients so that algae grow rapidly and algal blooms may form (Figure 5). The resulting rapid removal of carbon dioxide by photosynthesis can upset the bicarbonate–carbonate equilibrium and cause a pH rise, which in itself is damaging to the ecosystem.
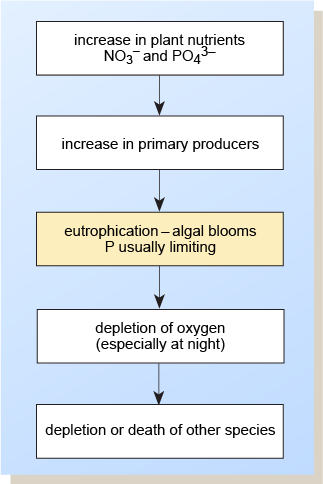
Figure 5 is a series of five rectangular boxes, one above the other, and connected by arrows pointing downwards. Starting from the top, they have the following wording: increase in plant nutrients, NO3− and PO43−; increase in primary producers; eutrophication – algal blooms, P usually limiting; depletion of oxygen (especially at night); depletion or death of other species.
In addition, at night the excess respiration (without the replacement of oxygen by photosynthesis) can deplete oxygen reserves, causing the death of higher organisms such as invertebrates and fish. This process can be compounded when algal blooms, through their decay, further reduce the oxygen content of the water.
In shallow water, the formation of benthic (bottom-living) mats of algae can create a smothering layer over sediments. This can hinder the supply of oxygenated water to eggs and impede the emergence of fry from salmon spawning grounds, for example.
Of the plant nutrients, the total inflow of phosphorus and nitrogen (especially at the productive time of year, spring and summer) is the most important. The main types of organism associated with algal blooms, and the different conditions needed for their continued growth, are as follows.
Blue-green algae (cyanobacteria)
Blue-green algae are able to fix atmospheric nitrogen and therefore are not limited by nitrate levels in the water. Neither are they dependent on dissolved carbon dioxide, because they can use the bicarbonate ions present. In addition, they are tolerant of relatively high pH. Their growth is limited by the phosphorus content.
Unicellular green algae
Green algae require nitrate, as they are unable to carry out nitrogen fixation. They also require fairly high levels of carbon dioxide, as they cannot use bicarbonate ions, and they are not tolerant of high pH values. Their growth is limited by both phosphorus and nitrogen.
Contact with or ingestion of blue-green algae can cause skin rashes, eye irritation, vomiting, fever, and pain in the muscles and joints, due to toxins produced by the algae. In addition, ‘red tides’ are harmful algal blooms that appear in coastal areas. They can produce toxic effects in humans, marine organisms and birds. The toxins produced may also make the surrounding air difficult to breathe (NOAA, 2013), and the bloom of algae often turns the water red.
Generally, increases in both phosphate and nitrate concentrations seem to be the most important factor in governing the rate of eutrophication in most waters. However, whether this is the only factor that limits the rate of eutrophication is still a matter of controversy. Any eutrophication is contingent on other aspects of the particular ecosystem such as the hardness, the pH value and the original distribution of algal species.
For freshwater plants, about eight times more nitrogen is required than phosphorus. Phosphorus thus limits eutrophication if nitrogen is more than eight times as abundant as phosphorus, while nitrogen limits eutrophication if its concentration is less than eight times that of phosphorus (UNEP, n.d.).
Nitrates can have a more significant effect on human health than phosphates if they are present in drinking water supplies.
- When nitrates are ingested by infants under six months of age, they can be converted to nitrite by bacteria in the digestive system. Nitrites combine with haemoglobin in the bloodstream, preventing it from carrying out its normal function of combining with oxygen and carrying it around the body. This can result in a serious, though rare, condition called methaemoglobinaemia (‘blue baby syndrome’), which can be fatal (Skipton and Hay, 1998).
- Ingestion of nitrate and nitrite by people with a low intake of vitamin C increases the risk of stomach cancer (Ward et al., 2011).
Water quality standards exist to control pollution of domestic water supplies. The maximum limit set by the EU for nitrates in drinking water is 50 g m−3 (as NO3−) (EU, 1998). Water with a high nitrate content can be treated for drinking using reverse osmosis.
The effects on the biota of organic pollution and artificial eutrophication are summarised in Figure 6. When organic pollution occurs, the main types of organisms present are the decomposers. In the case of eutrophication, the producers are dominant, and in larger quantities than in ‘clean’ waters.
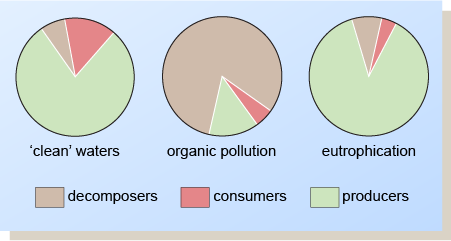
Figure 6 shows three circles representing clean waters, organically polluted water and water suffering eutrophication. The proportions of producers, consumers and decomposers in each are shown by means of slices (as in a pie) as follows (approximately):
| Clean waters | Organically polluted waters | Waters showing eutrophication | |
| Producers | 80 | 15 | 87 |
| Consumers | 13 | 5 | 4 |
| Decomposers | 7 | 80 | 9 |
5 Toxic pollutants
The term ‘toxic’ is a rather misused one. It is misleading to refer to one material as a toxic substance and to another as non-toxic without qualification. The toxicity of all materials depends on their concentration. Further complications may be introduced by the fact that some materials (e.g. selenium) are essential components of an animal’s diet, yet in anything other than very low concentrations they may have a toxic effect.
Other environmental factors to be taken into account include:
- the extent of biodegradation
- the rate of accumulation of a substance in the biota (and so within the food chain)
- the retention time of a substance within an organism.
An important mechanism for toxicity in the body is the poisoning of enzymes, which are the catalysts of all the bodily functions.
The terms used when explaining the effects of a toxic substance on an organism are:
- lethal – causing death by direct poisoning
- sublethal – not sufficient to cause death, but leading to a reduction in the number of species and/or individuals by, for example, causing a change in behaviour, growth or reproductive success
- acute – causing an effect (possibly death) within a short period of time
- chronic – causing an effect (lethal or sublethal) over a prolonged period of time
- accumulative – having an effect that is increased by successive doses.
To test the effects of toxicity, the LD50 (lethal dose) test is commonly used. The LD50 is the dose that is large enough to kill 50% of the sample of animals under test. Some examples of LD50 values for different chemicals are given in Table 1.
LD50 (mg per kg body weight) | Examples | Classification |
|---|---|---|
1–10 | arsenic | highly toxic |
10–100 | cadmium copper lead mercury | moderately toxic |
100–1000 | aluminium molybdenum zinc | slightly toxic |
>1000 | sodium iodine calcium potassium | relatively harmless |
Certain inorganic substances, such as cyanides, fluorides, sulfides, sulfites and nitrates, may be classified as toxic.
- Compounds of cyanide and sulfide interfere with the use of oxygen in respiratory reactions in cells.
- Excess fluoride can lead to mottling of teeth and bones in humans.
- Nitrates can cause ‘blue baby syndrome’.
However, the final effects of a toxic substance in water depend on environmental factors such as hardness, temperature and pH. Salts of heavy metals such as copper, silver, lead, gold, nickel, chromium, zinc, cadmium and mercury are toxic and will generally kill most aquatic organisms at very low concentrations, but they are generally less toxic in calcium-rich water (Wilson, 1988); a nickel–cyanide complex is 500 times more toxic to fish at pH 7 than at 8, because the complex dissociates into cyanide and nickel ions and a proportion of the cyanide forms the highly toxic undissociated hydrogen cyanide (HCN); and ammonia is 10 times more toxic at pH 8 than at 7 (EIFAC, 1968). Also an additive effect, synergism, may occur – for instance, evidence has been found for synergism between mercury and uranium (Sánchez et al., 2001).
Two particularly significant groups of toxic pollutants are the heavy metals, and synthetic organic substances such as some of the pesticides.
5.1 Pesticides
Pesticides are chemicals used to kill pests such as weeds, insects, fungi and rodents. After the Second World War, pesticides such as DDT (dichlorodiphenyltrichloroethane) – which were highly toxic, persistent and bioaccumulating – were commonly used in agriculture and for vector control (e.g. against the Anopheles mosquito, for malaria control). The populations of birds of prey declined due to eggshell thinning preventing the birth of live offspring. This was because of DDE (dichlorodiphenyldichloroethylene), a very stable metabolite of DDT (Faber and Hickey, 1973). Sexual development and behaviour in birds such as gulls was also disturbed (Fry and Toone, 1981). The use of DDT has now been banned in most countries, and the tendency is to opt for pesticides that degrade rapidly in the environment (FAO, n.d.).
Chemicals that exhibit the characteristics of DDT (i.e. high toxicity, persistence and bioaccumulation) are termed persistent organic pollutants (POPs), and most of the POPs are pesticides. Pesticides have been found to be carcinogenic in experimental animals, and therefore are possibly carcinogenic to humans. They are suspected of depressing the immune system, and of disrupting the endocrine system, mimicking or blocking normal hormone activity (Morner et al., 2002). More on endocrine disruptors is given below.
POPs can be transported by wind (e.g. from combustion and high-temperature processes such as those in the iron and steel industry) and water, and as such can affect areas far from the point of their use. Their persistence in the environment, and their potential to move up the food chain, led to the Stockholm Convention of 2001, under which nations agreed to reduce or eliminate the production, use and/or release of POPs (Stockholm Convention, 2008).
Organophosphates (such as malathion, diazinon and chlorpyrifos) are insecticides containing phosphorus. They act by inhibiting enzymes in the nervous systems of animals. Pyrethroids (synthetic versions of the short-lived natural pesticide pyrethrin, which is made from chrysanthemum flowers) are another category of insecticide, often used by householders to control pests such as leaf-eating insects and ants. Organophosphates and pyrethroids can attach to soil particles and get washed into rivers and streams, endangering aquatic life.
Amongst the POPs are polychlorinated biphenyls (PCBs), which are a group of organic chemicals used in a variety of ways (e.g. as hydraulic fluids, plasticisers, fire retardants, heat transfer fluids, paint additives, lubricants and cutting oils). Ingestion of water containing PCBs can lead to an increased risk of cancer, immune deficiency, and problems with the reproductive and nervous systems (EPA, 2012). Their use was banned in 1977.
The main problem with manufactured substances such as pesticides is that most are unknown in nature and thus organisms have not evolved to deal with many of them. Although some will be broken down into harmless substances by normal digestion processes, others will remain and accumulate in the organism exposed to them. This is known as bioaccumulation. If this organism falls prey to another organism, the toxic substances will be passed up the food chain and retained in increasing quantities within the bodies of organisms higher up the chain. The accumulation of toxic substances through the food chain is called biomagnification. An example of this was the case of Minamata disease, where consumption of fish contaminated by methyl mercury led to thousands of people suffering symptoms such as numbness in fingers and lips, difficulty in speech and hearing, inability to control their limbs, and seizures. There were many deaths, too (Porteous, 2008).
5.1.1 Endocrine disruptors
As mentioned above, pesticides can be endocrine disruptors. Many other chemicals are also classed as endocrine disruptors, i.e. they interfere with the synthesis, secretion, transport and binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis, reproduction, development and/or behaviour (Burkhardt-Holm, 2010).
Most endocrine disruptors are synthetic compounds (e.g. plasticisers such as bisphenol A, used in plastic bottles and food containers; sex steroids in contraceptive pills; paints; pesticides; alkylphenol polyethoxylates used as surfactants in detergents). They can end up in watercourses through sewage treatment works, surface water run-off, direct discharge or leachates from landfill sites. One effect that has been observed on wildlife is the feminisation of male fish (Jobling et al., 2002).
Having said the above, there are also natural sources of endocrine disruptors. For instance, Fusarium fungus infesting corn and other grains produces zearalenone, a potent oestrogenic chemical that causes cessation of lactation and hyperoestrogenisation in pigs (Burkhardt-Holm, 2010).
In terms of water supply, endocrine disruptors can be present if untreated groundwater is used for potable supplies, if the groundwater is contaminated with the suspect chemicals. Bottled water can contain endocrine disruptors from plasticisers and detergents used in the production process.
5.2 Acidity and heavy metals
Acidity can be detrimental to life forms. Table 2 shows the effects of low pH on fish.
| pH range | Effect |
|---|---|
| 6.5–9.0 | No effect |
| 6.0–6.4 | Unlikely to be harmful except when carbon dioxide levels are very high (1000 mg l−1) |
| 5.0–5.9 | Not especially harmful except when carbon dioxide levels are high (20 mg l−1) or ferric ions are present |
| 4.5–4.9 | Harmful to the eggs of salmon and trout species (salmonids) and to adult fish when levels of calcium, sodium and chloride are low |
| 4.0–4.4 | Harmful to adult fish of many types that have not been progressively acclimated to low pH |
| 3.5–3.9 | Lethal to salmonids, although acclimated roach can survive for longer |
| 3.0–3.4 | Most fish are killed within hours at these levels |
In addition, highly acidic waters can dissolve heavy metals, especially if the pH is below 3. This occurs in the case of mine wastewaters. The metals present tend to be brought into solution, especially iron, zinc, lead and molybdenum.
The information that is available on metal pollutants tends to refer to the total concentration of the metals; unfortunately this provides little information on a metal’s bioavailability (i.e. the ability of an organism to take up the metal) or how long the metal will stay in solution before it is removed into sediment. To be able to predict the adverse effects of metal pollutants in water it is necessary to determine the physical form or chemical speciation of the metal present, since metals can exist in a wide range of forms. The subject is too complicated to be covered fully here, but the following two theoretical examples are extreme cases of metal pollution to show the range of events that can occur.
- Example 1: After treatment to remove most of the lead, the effluent from a lead–acid battery factory is discharged into a river. The lead concentration in the effluent is still about 4 g m−3, with a trace of particulate lead in the form of lead sulfate. The lead sulfate settles out quickly. The soluble lead becomes attached to particles greater than 12 µm in size and settles out in the river. The discharge therefore causes a relatively small increase in the lead content of the river.
- Example 2: A sewage effluent is contaminated with cadmium from industrial sources. The cadmium is in the form of organic complexes formed from the organic-rich sewage in the sewage treatment process. In the river this form of cadmium does not settle out and is carried downstream over a long distance, remaining available to biological life throughout.
Metals dissolved in water can enter the food chain by a variety of mechanisms. For example:
- Phytoplankton absorb metals by diffusion across the external membrane.
Fish can take in metals by
- diffusion across the membrane of their gills
- ingesting metals, though metals taken in this way are not as readily bioavailable.
- Filter feeders such as oysters and cockles inhabit the surface of sediments and consume considerable amounts of particulate matter from the water that passes through them; they accumulate metals by ingestion.
6 Physical pollutants
Physical pollutants include:
- suspended solids
- immiscible liquids
- discharges that result in changes to the temperature or flow rate of the receiving water
- substances that impart a taste, odour or colour to the water.
6.1 Suspended solids
Various industries produce suspended material (or particulates) in their effluents, and this has several consequences.
All solids tend to reduce light penetration, so the growth of plant life in watercourses is inhibited. This will have secondary effects on food chains. Bottom-living animals and plants may be smothered as particles settle. If the particles settle on gravels, fish spawning can be seriously disrupted. Predators that hunt by day may be restricted in their activities: for example, in turbid water there may be an abundance of leeches as fish are no longer able to see and consume them as food.
One of the most important effects on animals is the damage to fish gills. Prolonged exposure to high levels of suspended solids (50 mg l−1 and above) is likely to lead to sublethal changes due to respiratory distress, and adverse growth and development (Au et al., 2004).
Some effluents pollute because substances in them enter into a chemical reaction with salts already dissolved in the water. For example, iron hydroxide may be precipitated if water containing iron is discharged into a naturally alkaline river. This phenomenon typically arises from abandoned mines, where the clear water pumped out and discharged into a clear stream can produce a bright orange coloration that prevents penetration of light and hence inhibits plant life.
Some suspended solids can also cause harmful effects when soluble toxic components present in them are dissolved into the water by biological or chemical action.
6.2 Immiscible liquids
Immiscible liquids may be present as oils, greases or tarry substances, often in the form of an emulsion (a colloidal suspension of one liquid in another, as in mayonnaise). They may affect turbidity in the same way as suspended solids. However, emulsions are not likely to settle to the bed of the river. Frequently they float on the surface and adhere to vegetation at the waterline. Some immiscible liquids are decomposed slowly by aquatic microorganisms. Many oils and tars are slightly soluble in water and thereby impart tastes and odours to it.
Oil is generally less dense than water and will spread over the surface to form an extremely thin, often visible film; a small quantity of oil is therefore likely to pollute a large area. Even when the oxygen demand in the water is low and oil imposes little additional biological load, the presence of an oil film with a thickness of only one thousandth of a millimetre (1 µm) may reduce the rate at which oxygen is transferred from air to water. It can also affect the life cycle of insects, since the larvae of some species float on the surface.
Oil is one of the more serious pollution problems. As an example, there are now around 3000 pollution incidents involving oil and fuels every year in England and Wales (Environment Agency, 2013). Although some of these affect land, the vast majority affect the water environment.
6.3 Discharges contributing to a temperature change
Industrial effluents are frequently discharged at temperatures different from those of the receiving river. Almost invariably the effluent is warmer than the river, since water is widely used for carrying away heat.
Within limits, a raised temperature increases the metabolic rates of all aquatic organisms. It also decreases the concentration of dissolved oxygen needed for saturation – for instance, the saturation concentration of oxygen in water at 5 °C is 12.79 g m−3, while at 15 °C it is 10.01 g m−3. The overall effect on the oxygen balance of a particular heated effluent therefore depends to a certain extent on the oxygen balance in the river at the point of discharge.
A small increase in the temperature of a clean, fast-flowing stream may not affect the ecosystem adversely. Provided oxygen is plentiful, plant and animal populations may be altered slightly but remain in a balanced state. Species indigenous to warmer climates may become established in a heated portion of a river. However, heated effluents are usually discharged to watercourses that are already polluted to some degree, so the polluting effects are compounded. A heightened biochemical oxygen demand (BOD) on the river water due to a sewage discharge upstream may be exacerbated by raising the temperature. Any animals or plants that die as a result of the heat or greater oxygen deficit are decomposed by bacteria, which decreases the oxygen level even more.
6.4 Discharges causing variations in flow rate
Variations in the flow of a river can result from excessive abstraction or from intermittent discharges of relatively large volumes of effluent, as when settling ponds (which are used to remove particulates from effluents in the ceramic industry, for example) are emptied. There are, however, maximum limits that must be adhered to.
Since the organisms that become established in a river will be those best suited to its conditions, sudden and repeated fluctuations in the rate of flow will mean that only those organisms that can withstand the changes will survive. Plants growing in silt deposits on the bed of a stream will be destroyed when the silt is washed away by a sudden increase in flow. When the flow falls, organisms that are dependent on a high dissolved oxygen concentration will die if the river reverts to a series of near-stagnant pools.
6.5 Substances causing taste, odour and coloration
Very low concentrations of some chemical compounds will produce unpleasant tastes and odours, or will taint the flesh of fish living in water contaminated by them. Interaction between substances may produce tastes that are apparent at concentrations well below those at which either substance is individually detectable. An ‘antiseptic’ taste of chlorinated tap water is obvious if the raw water supply contains phenols, since this results in the formation of chlorophenols. (Phenolic compounds can occur naturally in lowland rivers.) Unpleasant tastes and smells, usually earthy or sulfurous in nature, can also occur naturally from decaying vegetation.
The ecological effect of colour will depend on its light-absorptive properties in relation to the spectral requirements of algae and plants (i.e. which wavelengths of light they need). Many rivers are naturally coloured (e.g. those draining peat are light brown due to humic and fulvic acids) and yet are able to support biota, including trout. The ecological effects of colour are usually minimal compared with other factors.
Acivity 2
Each hour, an industrial plant discharges 600 m3 of treated effluent at 40 °C into a river that has an annual average temperature of 15 °C. The flow rate of the river is a constant 20 000 m3 per day.
- a.Assuming perfect mixing, what will the temperature in the river be after entry of the effluent, assuming no temperature loss to atmosphere?
- b.What impact will this have on the river?
Answer
a.The effluent flow rate is 600 m3 per hour, which equates to 14 400 m3 per day. The final temperature in the river will be:
b.The higher temperature will:
- increase the metabolic rate of the aquatic organisms in the river
- decrease the level of dissolved oxygen, affecting organisms and plants in the water
- cause species more suited to waters warmer than the ambient to become established
- prevent fish from migrating upstream past the entry point of the heated effluent.
7 Biological pollutants
Biological pollutants are organisms that may be harmful to other forms of life, but they have to be ingested to have any effect. The most usual form of transmission is the faecal–oral route, in which faecal matter from one human being finds its way into another. This can happen through the ingestion of faecally contaminated water or food. Alternatively, sometimes pathogenic organisms can be consumed through eating contaminated undercooked food, as in the case of certain strains of Escherichia coli.
The effects of the different organisms are varied, and can be more easily ascertained through sources such as the WHO or local health protection agencies. However, in this subsection I will describe the characteristics of the main water-borne pollutants, namely:
- pathogenic bacteria
- coliforms
- faecal streptococci
- Clostridium perfringens
- viruses
- protozoa
- helminths
- other biological pollutants.
7.1 Pathogenic bacteria
As well as the bacteria that are found naturally in river water and that are essential for the natural cycle of nutrients, there may be other, less desirable bacteria. Pathogenic bacteria (such as Salmonella typhi, Figure 7) can cause disease in a variety of organisms, including humans. Since the presence of pathogenic bacteria is generally due to the activities of humans, it constitutes a form of pollution. Non-pathogenic bacteria, by definition, are harmless; indeed, as already mentioned, they can be beneficial and form an essential part of the aquatic ecosystem.
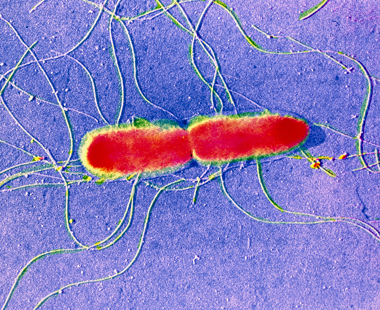
Figure 7 shows a single Salmonella typhi, which looks like two rectangular tablets joined together at the ends, with long hair-like tendrils emanating from them.
Effluents from sewage treatment works contain some pathogenic bacteria, but in far smaller numbers than in incoming sewage since the sewage treatment processes will generally eliminate more than 99% of them.
Since pathogenic bacteria are accustomed to human body temperature (about 37 °C), they do not flourish in river water and die off relatively quickly.
7.2 Coliforms
Coliforms are a large group of bacteria, often of intestinal origin. The coliform Escherichia coli (Figure 8) is present in the intestines of humans and other mammals. Its presence in water implies that human pathogens from faeces may also be present, and it is therefore a useful indicator organism of faecal contamination.
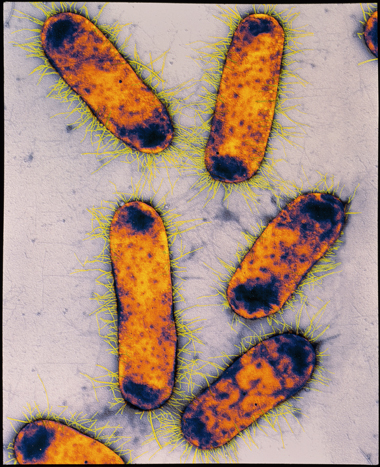
Figure 8 shows several E. coli, which look like rectangular tablets with short hair-like tendrils.
E. coli is a rod-shaped faecal coliform about 0.5 μm by 2–3 μm in size. The strain of E. coli usually present in humans is harmless and occurs consistently in faeces in far greater numbers than pathogenic bacteria. However, there are other strains – such as E. coli O157:H7 – that are pathogenic. These have been found in partially cooked meat and have led to deaths (Rangel et al., 2004).
Concentrations of E. coli as low as 10 cells per litre can be detected. The presence or absence of E. coli in a water sample provides an important indicator of pollution and possible risk to public health.
7.3 Faecal streptococci
The faecal streptococci group of bacteria consists of the species Streptococcus faecalis (Figure 9), S. faecium, S. durans, S. equinus and S. bovis. The bacteria are approximately 1 µm in diameter and occur in chains of varying length. They die fairly quickly outside their host, so their presence is indicative of recent pollution.
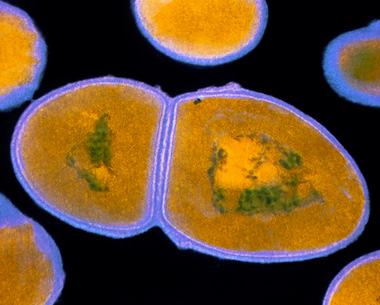
Figure 9 shows a single Streptococcus faecalis, which looks like a shoe-print but with the bottom (heel) part only slightly smaller than the front part.
7.4 Clostridium perfringens
C. perfringens (Figure 10) is an anaerobic organism present in the intestines of humans and animals at much lower numbers than E. coli. It is a common cause of food poisoning. The cells are rod-shaped (about 5 µm by 1 µm) and can form endospores. They can cause severe abdominal cramp and diarrhoea if present in water that is ingested.
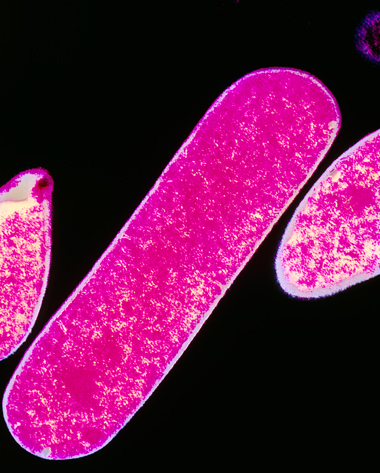
Figure 10 shows a single Clostridium perfringens, which looks like a rectangular tablet.
Endospores – or spores, as they are commonly referred to – are hardy structures that certain bacteria can form when their environment becomes unfavourable for growth. The purpose of the endospore is survival; it is very resistant to heat and desiccation, and may survive for many years at normal temperatures. When environmental conditions are favourable for growth, the endospore reactivates to form a normal, single cell. This ability leads to C. perfringens being present in unchanged numbers long after other faecal indicators have died out. Thus its presence in the absence of E. coli indicates intermittent faecal contamination.
7.5 Viruses
Viruses are tiny (5–30 nanometres in size) infective agents that can grow only in living cells. The virus that causes smallpox, declared eradicated from the world in 1979, is illustrated in Figure 11.
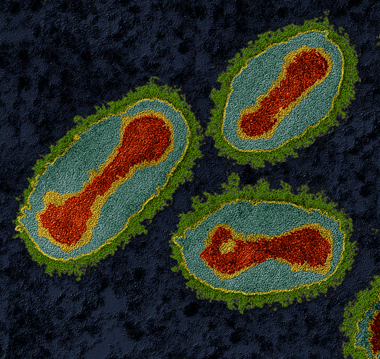
Figure 11 shows several smallpox viruses, which look oval in shape with a dumbbell-shaped structure inside.
Most viruses are able to remain viable in water at low temperatures, provided there is some organic matter present. Once excreted, the number of viruses cannot increase since they only multiply within living susceptible cells.
The main threat to water quality comes from human enteric (intestinal) viruses that are produced by infected persons and excreted faecally. Depending on local circumstances, this may contaminate river water directly or, if treated, via sewage effluent that may be discharged into a river. If the river water is abstracted and treated for drinking purposes, the viruses may not be completely removed. (It is possible for a person to be susceptible to only one viral particle.)
The presence of any enteric virus can be taken as an indication of the possible presence of other harmful viruses. In temperate climates, enteric viruses occur at peak levels in sewage during late summer and early autumn. The exception is the hepatitis virus, which increases in the colder months.
7.6 Protozoa
There have been several outbreaks of protozoal infections from water in several countries. For instance, each year in the UK there are 3000–6000 confirmed cases of cryptosporidiosis, caused by the protozoa Cryptosporidium; the largest outbreak was in Torbay, Devon in 1995, when 575 people were taken ill (Hunter et al., 2003).
The most common symptom of cryptosporidiosis is watery diarrhoea. Other symptoms include stomach pain, nausea, vomiting, fever and weight loss (Centers for Disease Control and Prevention, 2010). In addition, some people can act as carriers of Cryptosporidium.
Another, similar condition is giardiasis, caused by the protozoa Giardia. Giardiasis outbreaks are not common in the UK, but one occurred in Bristol in 1985, when 108 cases were diagnosed (Jephcott et al., 1986).
Techniques for sampling and analysis of Cryptosporidium are complicated and time-consuming, requiring the filtration of large volumes of water (100–1000 litres), followed by several stages of elution, isolation and concentration of the oocysts, and then identification and enumeration by immunofluorescent microscopy. Initial testing does not provide information on whether the oocysts are viable and therefore capable of causing disease – this requires further testing. As a result, there is no specific standard for the organism in EU or UK regulations. There is, however, a general requirement that drinking water should not contain any microorganism or parasite at a concentration that would constitute a potential danger to human health (Water UK, 2011).
Cryptosporidium and Giardia can be trapped by membrane filtration or slow sand filters. Other types of filters, such as wound fibre filters, are also employed. Chlorination and UV radiation at normal doses are ineffective against these organisms.
7.7 Helminths
Helminths (Figure 12) are parasitic worms that can cause ill health in humans. They range from a millimetre long to more than a metre (Baron, 1996).
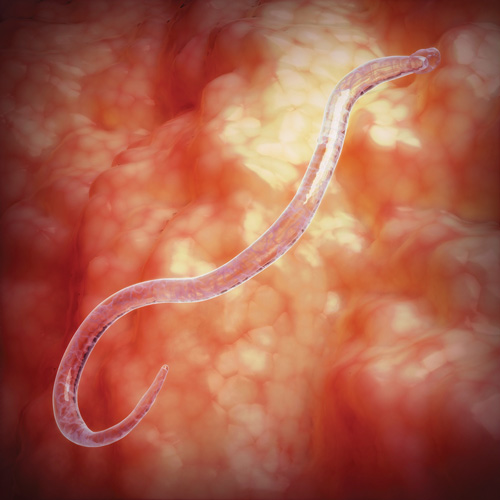
Figure 12 shows a helminth, which looks like a long translucent worm.
Helminths can cause morbidity, and sometimes death, by compromising the nutritional status of the infected person. They can also affect cognitive processes, induce tissue reactions and provoke intestinal obstruction or rectal prolapse (WHO, 2013).
Infection occurs through ingestion of helminth eggs that are present in food. For example, helminth eggs may be present in the meat of cattle grazing on land that is contaminated by poorly treated sewage effluent or sludge.
7.8 Other biological pollutants
Many other forms of pollution mentioned previously could be considered as biological pollutants, e.g. algal blooms and the growth of sewage fungus. Examples that are more clearly of this type of pollution are the many species of blue-green algae, which produce substances that are toxic to terrestrial organisms and can impart tastes and odours to water.
Activity 3
a.
a. Continuously flowing organic pollutants such as domestic sewage cannot cause long-term damage to a watercourse in the way that toxic pollutants can.
b.
b. A temperature rise in a waterway leads to higher productivity but doesn’t affect the BOD.
c.
c. Effluents from fish farms can be badly polluted. Concern has arisen about these effluents mainly because they contain unconsumed food and faecal matter from fish.
d.
d. Pesticide pollution of watercourses is due solely to large-scale use of these compounds by farmers.
e.
e. Metals that are water soluble are able to bioaccumulate by diffusing across the biological membranes of organisms.
The correct answers are c and e.
Answer
Statement (e) is true. Statement (c) is also true, though concern has also arisen because fish farm effluents contain antibiotics (see Section 2).
Statement (a) is false: heavy organic loads on a watercourse can cause long-term damage to the water in the vicinity of the effluent outfall. However, recovery is more likely if pollution ceases than would be the case for toxic chemicals.
Statement (b) is false: the BOD will increase, as the metabolic rates of the microorganisms will be higher at a higher temperature.
Statement (d) is false: householders use a large quantity of pesticides in the form of herbicides and insecticides, and these can also contribute to water pollution.
Activity 4
a.
a. The toxicities of individual chemicals in a mixture can be summed up to give an overall toxicity figure. This is simpler than testing a complex mixture.
b.
b. In toxicity tests for a proposed discharge, the most sensitive species in the receiving watercourse should be used as the test organism.
c.
c. When suspended solids settle on the river bed, it is only benthic plants that are affected, as the light will not reach the leaves.
d.
d. E. coli is a species of bacterium that inhabits human intestines and is always harmless.
e.
e. Clostridium perfringens is a pathogenic bacterium that lives in the human gut and can cause food poisoning.
f.
f. Controlling the discharge of the culprit chemicals from industry will overcome the problem of endocrine disruptors.
The correct answers are b and e.
Answer
Statements (b) and (e) are true.
Statement (a) is false: there may be synergistic effects between the individual chemicals in the mixture.
Statement (c) is false: fish spawning and the ecology of invertebrates are also disrupted.
Statement (d) is false: certain strains of E. coli (e.g. E. coli O157:H7) are pathogens and can cause fatalities.
Statement (f) is false: the discharge of oestrogens, often at high levels, due to the use of contraceptive pills will still contribute to the problem.
Activity 5
Indicate the causative agent (virus, bacterium, protozoan or helminth) of each of the following diseases:
- cholera
- poliomyelitis
- typhoid
- bilharzia
- anthrax
- cryptosporidiosis.
Answer
| Cholera | bacterium |
| Poliomyelitis | virus |
| Typhoid | bacterium |
| Bilharzia | helminth |
| Anthrax | bacterium |
| Cryptosporidiosis | protozoan |
7.9 Typical pathogens
Table 3 lists some of the major pathogens that cause disease to humans and are likely to be found in polluted water.
| Organism type | Organism | Disease | Remarks |
|---|---|---|---|
| Bacteria | Vibrio cholerae | Cholera | Transmitted via sewage and polluted waters in cholera-endemic areas |
| Salmonella typhi | Typhoid fever | Common in sewage | |
| Salmonella paratyphi | Paratyphoid fever | Common in sewage | |
| Salmonella spp. | Food poisoning | Cause of food poisoning; usually found in contaminated food of animal origin | |
| Shigella spp. | Bacillary dysentery | Polluted waters are main source of infection | |
| Bacillus anthracis | Anthrax | Can be found in effluents from tanneries processing hides from infected animals; spores resistant to treatment | |
| Brucella spp. | Brucellosis (Malta fever) in humans; contagious abortion in sheep, goats and cattle | Normally transmitted by infected milk or by contact | |
| Leptospira icterohaemorrhagiae | Leptospirosis (Weil’s disease) | Carried by sewer rats; also present in water contaminated by urine from infected animals and humans; can infect through cuts in skin, or intact skin if immersed for a long time | |
| Viruses | Poliovirus | Poliomyelitis | Transmitted by faecal–oral route via contaminated food or water |
| Hepatitis A virus (HAV) | Hepatitis A | Transmitted by faecal–oral route via contaminated food or water | |
| Protozoa | Entamoeba histolytica | Amoebic dysentery | Spread by contaminated waters and sludge used as fertiliser; common in warm countries |
| Giardia lamblia | Giardiasis | Found in inadequately treated water | |
| Cryptosporidium spp. | Cryptosporidiosis | Carried by agricultural livestock and infected persons | |
| Helminths | Taenia saginata | Tapeworms | Eggs very resistant, present in sewage sludge and sewage effluents; can be present in contaminated water sources and food |
| Ascaris lumbricoides | Nematode worms | Present in sewage effluents and dried sludge used as fertiliser | |
| Schistosoma haematobium, Schistosoma mansoni | Bilharzia | Carried by water snails in rivers and irrigation ditches contaminated by human waste in specific regions of the world; enter humans by direct penetration of skin |
Conclusion
Water is crucial for our survival. It is used in our bodies for a number of purposes:
- cooling
- as a waste disposal medium
- as a conductor for nerve impulses
- as a component in the digestion of food
- as a solvent in which chemical reactions take place.
The hydrological cycle is the continuous cycling of water between land, water surfaces and the sea. Pollutants entering a river can be washed away to sea, or degraded by microorganisms present in the river. Excess pollution in a river can damage the plant and animal life present in the river by reducing the oxygen content of the water.
Point sources of pollution are discharge points where pollutants collected by a network of pipes or channels are released. Diffuse sources, on the other hand, are characterised by multiple discharge points that cannot be located exactly. Point sources can be easily controlled, while diffuse sources pose great difficulty in terms of collection and control.
Lakes are much more prone than rivers to pollution as they do not have the flushing effect of rivers. They also do not have the dilution effect of large bodies of water such as the sea. Eutrophication can be a particular problem for lakes.
The major sources of water pollution are:
- discharges from sewage works, often containing industrial wastes
- discharges from manufacturing and industrial plants, including mines
- discharges from animal rearing, fish farming and agriculture
- seepage from domestic and industrial landfill sites
- urban surface water run-off.
Different pollutants affect the aquatic environment in different ways. While at low concentrations many pollutants (e.g. organic materials, N and P) may be beneficial, at high levels they can adversely affect the ecology of the system. Excess nitrate can be particularly harmful to babies.
Many of the toxic pollutants in effluents are synthetic, and therefore do not easily biodegrade naturally.
The effects of physical pollution on the ecology of a river system can be complex, affecting the feeding and breeding habits of the different species.
Biological pollutants can spread disease through water, and also disrupt the ecology.
The measurement and control of water quality is therefore of crucial importance in the interests of public health and the maintenance of the environment.
Table 4 gives a summary of the effects of the different pollutants discussed in this unit.
| Pollutant | General effect | Effect on biota | Effect on water supplies | Sources: natural | Sources: result of human activity |
|---|---|---|---|---|---|
| Organic (biodegradable wastes) | Increased oxygen demand; food provided for organisms lower down in food chain | Tolerated in moderate quantities if release not too quick, serious if dissolved oxygen (DO) drops too quickly | Increased need of treatment | Run-off and seepage through soil | Domestic sewage, food processing, animal wastes |
| Plant nutrients | Excessive plant growth | Demand on DO | Increased need of treatment | Natural degradative processes | Animal wastes, fertilisers, detergents, industrial wastes |
| Toxic chemicals (e.g. heavy metals, pesticides, phenols, PCBs) | Toxic to humans, animals and plants | Could be lethal | Increased need of treatment or control | Rare | Detergents, pesticides, tanneries, pharmaceuticals, wool scouring, refineries |
| Endocrine disruptors | Alteration of ecology | May adversely affect health and reproduction of humans and animals | Can be present in water sold in plastic bottles | Fusarium species of fungus | Chemical manufacture, intensive farming |
| Acids/alkalis | Lowering/raising of pH; acids can dissolve heavy metals | Only narrow range of pH tolerable for most plants and animals; heavy metals toxic | Corrosion | Naturally acid or alkaline rock | Battery, steel, chemical and textile manufacturing; coal mining |
| Suspended solids | Reduction in light penetration (increased turbidity), blanketing, introduction of colour | Photosynthesis reduced; blanketing of benthic plants and animals; obstruction of gills of fish | Obstruction of filters; increased need of treatment | Soil erosion, storms, floods | Pulp mills, quarrying, any building or development work involving ground disturbance |
| Immiscible liquids | Formation of a layer at the water surface that could prevent O2/CO2 interchange | Reduced DO; insect breeding affected | Interference with treatment processes | Unlikely | Oil-related activity |
| Heat | Decrease in DO; increase in metabolic rate of aquatic organisms | Possible reduced breeding or growth of aquatic organisms | None | Unlikely | Power plants, steel mills |
| Taste-, odour- and colour-forming compounds | Taste, malodour, colour | Tainting of fish | Increased need of treatment | Peat | Chemical manufacture or processing |
| Microorganisms | Pathogenic to humans | None | Increased need of treatment | Animal excrement | Contamination from human wastes |
Glossary
- algal blooms
- A rapid increase in the population of algae in an aquatic system.
- bioaccumulation
- The accumulation of a substance, such as a pesticide or another organic chemical, in the tissues of a living organism.
- bioavailability
- Of a substance, the degree to which or rate at which that substance is absorbed or becomes available at the site of physiological activity, i.e. the extent to which it can be taken up by living organisms.
- biomagnification
- The sequence of processes in an ecosystem by which higher concentrations of a particular chemical are reached in organisms higher up the food chain.
- catalyst
- A substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change.
- chemical speciation
- The chemical form or compound in which an element occurs in both non-living and living systems.
- colloidal
- Term referring to a system in which finely divided particles, which are approximately 10 × 10−10 m to 10 000 × 10−10 m in size, are dispersed within a continuous medium in a manner that prevents them from being filtered easily or settled rapidly.
- diffuse sources
- Sources of pollution (often small) that have no specific point of discharge.
- diffusion
- The movement of atoms or molecules from an area of high concentration to one of low concentration.
- dissociates
- In reference to ionic compounds (complexes or salts), to separate or split into smaller particles, ions or radicals, usually in a reversible manner.
- emulsion
- A mixture of two or more liquids that are normally immiscible.
- enzyme
- A substance produced by a living organism that acts as a catalyst to bring about a specific biochemical reaction.
- homeostasis
- Maintenance of a constant internal environment.
- hydrological cycle
- The natural water cycle that describes the continuous movement of water on, above and below the surface of the Earth.
- nitrogen fixation
- The process by which nitrogen gas (N2) in the atmosphere is converted into ammonia (NH3).
- persistent organic pollutants
- (POPs) Organic compounds that are highly toxic, persist in the environment, bioaccumulate in human and animal tissue, and can be transported by wind and water. Most POPs are pesticides.
- point sources
- Single identifiable sources of pollution from which pollutants are discharged, such as a pipe.
- synergism
- Condition in which the interaction of two or more substances results in a combined effect that is greater than the sum of their separate effects.
References
Acknowledgements
This course was written by Suresh Nesaratnam.
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this course
Course image: Kris Krug in Flickr made available under Creative Commons Attribution-NonCommercial-NoDerivs 2.0 Licence.
Grateful acknowledgement is made to the following sources.
Figure 1: Cartoon by Ajit Nunan. Courtesy of the Centre for Science and Environment, New Delhi.
Figure 3: Department of Geology and Geography, Ohio Wesleyan University.
Figures 7, 9 and 10: Copyright © CNRI/Science Photo Library.
Figures 8 and 11: Copyright © Eye of Science/Science Photo Library.
Figure 12: Copyright © RGB Ventures LLC dba SuperStock/Alamy.
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
Copyright © 2016 The Open University