Living without oil
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Saturday, 4 May 2024, 4:19 PM
Living without oil
Introduction
Crude oil is currently our most important global source of energy. It is vital in the manufacture of many modern materials. But the world's supply of oil is finite, its price is unstable and our reliance on oil has damaging environmental consequences. This course explains why developing alternatives to oil is an essential and urgent task for humanity.
This OpenLearn course provides a sample of level 1 study in Science.
Learning outcomes
After studying this course, you should be able to:
demonstrate knowledge and understanding of the core concepts, principles and language relating to the science and social issues appropriate to the development of alternatives to oil products
demonstrate a knowledge and understanding of the impact of the use of oil on the future of our planet
express and rewrite concepts in an objective and factually correct way
make sense of information presented in different ways, including textual, numerical or graphical material.
1 Introducing oil
Over the last 100 years or so, humanity has come to rely increasingly on oil products. Oil is so central to modern life that it's no exaggeration to say that, at the moment, modern economies could not operate without vast supplies of crude oil.
From your general knowledge, what are the two major uses of oil in the modern world and why do we need to consider 'living without oil'?
The two major uses of oil are: (i) to produce fuels for transporting people and goods, and (ii) as the raw material needed to manufacture a huge variety of modern materials. Oil may need to be replaced because the supplies of oil are limited and there are concerns that it will run out. Additionally, burning oil products as fuel produces carbon dioxide and so contributes to anthropogenic ('human-made') global warming.
The central contention of the Living without oil course is that developing alternatives to oil is an essential and urgent task for humanity. Those alternatives will necessarily have to involve both new sources of fuel and new technologies for transport, and new ways to produce modern materials.
So in order to set the scene, this course will address in more detail the two questions: why is oil important, and why do we need to replace it?
A note about terminology
Chemists may argue that this course should have a more precise title! The term 'oil' can have a number of related meanings - in general the term can be used to refer to any thick liquid that doesn't mix with water. In everyday language, the term 'oil' is used to refer to petroleum (crude oil), which is indeed a thick liquid that doesn't mix with water. Chemists sometimes refer to crude oil as 'mineral oil' in order to distinguish it from oils derived from plants or other sources. In this course the term 'oil' will be used in its everyday sense - meaning petroleum (crude oil).
1.1 Oil consumption
One way of establishing the importance of oil in the modern world is simply to look at the numbers. Oil fuels an estimated 95% of the world's transport. In 2010 the world consumed a staggering 12 million tonnes of oil daily, which is roughly equivalent to 14 billion litres of oil every day. These are enormous quantities - equivalent to the volume of over 5000 Olympic swimming pools or filling enough road tankers to stretch the distance from London to New York (Figure 1).
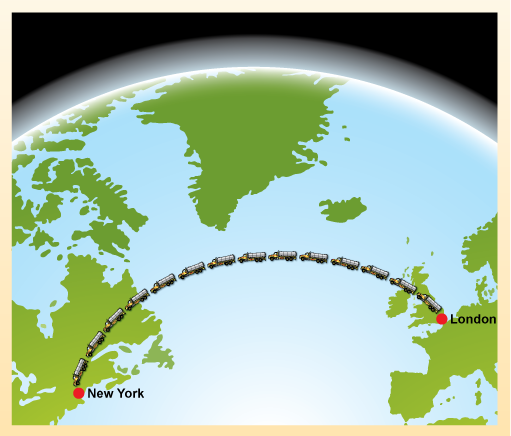
Part of a globe of the Earth, showing the North Atlantic, with London and New York marked with red spots, and an arc of 14 oil tanker lorries stretching between them.
In 2010 the UK alone was responsible for the consumption of 92.3 billion litres of oil. It can be difficult to imagine what a large quantity like 92.3 billion litres represents, so it's useful to convert this figure into something more meaningful - in this case, you will calculate the average daily consumption of oil for each person in the UK.
When performing calculations involving very large numbers, like those involved when estimating oil consumption, scientists tend to write numbers in a specific way - using scientific notation. In scientific notation, 92.3 billion litres is written as 9.23 × 1010 litres. (One billion is 1 × 109, so 92.3 billion becomes 92.3 × 109, which is written in formal scientific notation as 9.23 × 1010.)
The UK population in 2010 was approximately 62 million. Express the UK population in scientific notation.
6.2 × 107 people (one million = 1 000 000 = 1 × 106)
Setting out your calculation using scientific notation, calculate the number of litres of oil consumed on average by each UK resident on each day in 2010.
The average annual consumption of oil for each person
= (9.23 × 1010 litres per year)/(6.2 × 107 people)
= 1.49 × 103 litres per year per person
Converting this to a daily consumption:
= (1.49 × 103 litres per year per person)/(365 days per year)
= 4.1 litres per person per day
A typical adult needs to ingest up to 3 litres of water in drinks and food each day - so it could be argued that a typical UK adult is responsible for the consumption of more oil than drinking water!
In assessing the importance of oil and its potential replacements, it's inevitable that some numerical comparisons have to be made. This book will include a small number of calculations - in particular, it will be important at times to be able to convert quantities quoted in one set of units into another.
The oil industry tends to measure the quantity of oil in barrels - a rather old-fashioned unit, given the fact that oil is no longer transported in barrels. A careful web search for the world consumption of oil might lead to the annually published BP Statistical Review of World Energy (the source of most of the data in this section), where the value quoted will always be in millions or billions of barrels of oil. The daily global consumption of oil for 2010 is given in the BP Statistical Review as 87 million barrels of oil per day. The price of crude oil is generally given as the price (in US dollars) per barrel. On the other hand, scientists internationally tend to use SI units, where SI is an abbreviation for the Système International d'Unités (International System of Units). Examples of SI units are the kilogram or tonne for mass and the litre for volume. This course uses SI units wherever possible, but on occasions it will be necessary (or just more convenient) to go along with the oil industry and quote values in barrels.
In order to translate the units used in oil industry publications to those more commonly used in scientific publications, you need to be able to convert 'barrels' into standard SI units of mass and volume.
Converting quantities of oil quoted in barrels to litres is relatively straightforward, since one barrel of oil contains a volume of 159 litres. To convert from barrels to tonnes is slightly trickier as it depends on where the oil comes from. Crude oils from different sources have different densities but, on average, one barrel is equivalent to 0.14 tonnes of oil.
Express the UK's annual oil consumption for 2010 in barrels, and estimate what the mass of that oil might be in tonnes.
The 2010 annual consumption of oil for the UK was 9.23 × 1010 litres.
Annual consumption in barrels = (9.23 × 1010)/159 = 5.81 × 108 barrels
Estimated annual consumption in tonnes = 5.81 × 108 × 0.14 = 8.1 × 107 tonnes
Crude oil in its unrefined state is not very useful - it has to be processed to produce the fuels and materials so essential in the modern world. However, for now, you just need to be aware that each barrel of oil undergoes extensive refining and processing to produce fuels and materials. You estimated that each person in the UK consumes around 4 litres of crude oil each day - in the following sections you will explore what that 4 litres is used for.
A note on sources
The statistical data quoted in this section comes from the annually published BP Statistical Review of World Energy which was mentioned above. This is widely accepted as a reliable source, though there are those who have challenged its trustworthiness. Academic publications indicate the sources of the information they have drawn on by using references. This course does not reference every source of information used in the text, as this might distract from the flow of the material.
Activity 1 Living without oil
The estimated time for this activity is 15 minutes.
Watch the 3-minute video clip in which some of the central issues surrounding living without oil are introduced and then answer the questions below. If you have time, you may wish to watch the whole clip through once, and then read the questions before watching the video again. Use the pause button while noting down your answers to each question.
Transcript: Video 1
Question
- a.The clip states that 'the extraction and use of oil have damaging impacts on local environments and global climate'. Give one example each of
- i.how the extraction of oil has damaged a local environment
- ii.how the use of oil damages local environments
- iii.how the use of oil has a damaging impact on the global environment.
- b.What alternatives to oil as a source of transport energy feature in the video?
- c.There are three broad alternatives to oil-based transport: biologically derived liquid fuels (biofuels), hydrogen and battery electric vehicles. The video makes it clear that all these technologies are likely to be tested in the near future. Which of these, if any, do you personally think is most likely to feature in meeting our transport needs in the long term?
Answer
a.
- i.The extraction of oil can damage local environments through oil spillages; one example would be the Deepwater Horizon oil spill (Section 6.2), though there are many others.
- ii.The use of oil can damage local environments through air pollution; one example you might mention is the role that particulates from diesel engines play in smog formation, though again you may have chosen other examples (Section 8).
- iii.The most damaging impact that the use of oil has on the global environment is its contribution to anthropogenic global warming (Section 7).
- b.The alternatives to oil (which all feature in the video) are:
- getting oil (biofuels) from algae
- converting used cooking oil to biodiesel
- using hydrogen-fuelled cars
- battery electric vehicles
- c.Your answer will depend on your point of view, and your view may change. For my part (John Baxter), my experience in writing the course has led me to feel that perhaps battery electric vehicles may dominate the long-term future of transport - but I am far from sure!
2 Oil for personal transport

Part of one carriageway of a motorway showing a traffic jam of cars and lorries.
Most obviously, oil products provide us with the energy to move us around (Figure 2) and a proportion of the 4 litres of crude oil each person consumes each day will be used to fuel personal transport. Several crude oil products are used to fuel transport. Petrol (called gasoline in some parts of the world) and diesel are the fuels primarily used for road transport, whilst kerosene is used to fuel jet aircraft.
You can start to estimate how much of the 4 litres each person consumes is used for personal road-based transport by roughly estimating how much fuel you use for personal transport each day. You could begin by trying to select a typical day and estimating how much fuel was consumed on that day; but transport needs vary so much from day to day, that it is more appropriate to look at a longer period, and take an average. One way to do this would be to look at the transport fuel consumed over a period of a week: Table 1 shows estimates of the amount of fuel (petrol) used for car transport in a relatively quiet week for the author.
| Day | Journey destination | Length of return journey/km | Estimated volume of petrol consumed/litres |
|---|---|---|---|
| Sunday | cinema | 30 | 1.8 |
| Monday | office | 40 | 2.4 |
| Tuesday | train station | 30 | 1.8 |
| Wednesday | office | 40 | 2.4 |
| Thursday | office | 40 | 2.4 |
| Friday | office | 40 | 2.4 |
| Saturday | supermarket | 5 | 0.3 |
| TOTAL | 225 | 13.2 |
In general, UK petrol engine cars have an average fuel consumption in the region of 17 kilometres per litre (km l−1), which is the figure that has been used in the calculations in Table 1. Diesel engines tend to be more efficient and deliver an average of around 23 km l−1.
Here scientific SI units, km l−1, are used rather than miles per gallon which might be commonly used in the UK. Expressing kilometres per litre in the form km l−1 is again a standard scientific way of expressing things.
Here is an example of the type of calculation used to generate the final column in Table 1.
Approximate distance to cinema and back = 30 km
Estimated fuel efficiency = 17 km l−1
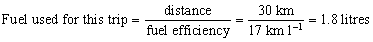
Question 1
Try to estimate how many litres of petrol or diesel the main car user in your household has consumed in the last week. Make a list of the journeys made, estimating the total distance covered in kilometres. Don't worry about being exact - you are just trying to get a rough idea. If you have a small petrol engine car, you can assume that your fuel efficiency is 20 km l−1; if you have an average-sized car, assume a fuel efficiency of 17 km l−1; and for a larger car, assume 14 km l−1. If you have a diesel engine car, assume a fuel efficiency of 23 km l−1. If you have estimated your distances in miles (or read them in miles from the display in your car), then you will need to multiply by 1.6 to convert miles to kilometres.
If no one in your household uses a car, try to estimate how much petrol you would have used if you replaced your usual modes of transport with a small petrol engine car.
Answer
The answer for the author is in Table 1 and the associated text. Your answer will be personal to you.
Question 2
After processing, a typical litre of crude oil will yield around 0.46 litres of petrol, 0.23 litres of diesel and a variety of other products. Calculate how many litres of crude oil would have to be processed to supply your house-hold with fuel for the car for an average day. You should assume that the last week is a typical one. Once again, if no one in your household uses a car, estimate how much petrol you would have used if you replaced your usual modes of transport with a small petrol engine car.
Answer
The author's answer would be as follows:
Daily petrol consumption ![]()
![]()
= 1.9 litres approximately
A typical litre of crude oil will yield around 0.46 litres of petrol, so the number of litres of crude oil needed to generate 1.9 litres of petrol would be given by:
litres of crude oil processed = 1.9/0.46
= 4.1 litres
This answer is discussed in the text that follows.
Table 1 and the answer to Question 2 imply that the author's car is responsible for the consumption of 4.1 litres of crude oil a day. Three people live in the author's household and share the use of the car, so arguably the car's consumption can be divided between three, so the car alone accounts for about 1.4 litres of the 4 litres of crude oil each person in the author's one car household might be expected to consume each day. Coincidentally the author's calculations imply that in his household his car consumes the same amount of crude oil each day (4.1 litres) as the average UK citizen. Your calculations may give a quite different figure, depending on the car and the distance travelled.
The week shown in Table 1 is not a typical one for the author's family as the annual mileage recorded for his car is in the region of 10 000 miles or approximately 16 000 kilometres. This suggests that the car is responsible for not 1.4 but around 1.9 litres of the 4 litres of crude oil each of the three people in the household might be expected to consume each day. (You may like to check this calculation for yourself.) These are very approximate calculations, but they demonstrate that personal road transport is responsible for a very significant proportion of the crude oil consumed in the developed world. The calculation did not take into account trips made by public transport which also will be responsible for a small proportion of our daily consumption of crude oil.
Why is the consumption of crude oil in public transport likely to be much less significant in determining our individual consumption of crude oil?
Public transport carries many more passengers than cars can, so the consumption of crude oil by a bus or train will be spread across many more individual passengers.
Other forms of transport are important too - aviation is a huge consumer of fuel derived from crude oil (largely kerosene). A single return flight to New York from London could easily triple an individual's annual personal consumption of crude oil products.
However, as important as personal transport is in determining the amount of crude oil we consume, to gain a full appreciation of the centrality of oil in the modern world you also need to look at the role crude oil plays in transporting goods.
3 Virtual oil
There may be those amongst you who have used no powered transport at all in the last week - perhaps you cycled, walked or ran everywhere you needed to go, or even rode a horse. However, in the developed world, even individuals who don't personally use powered transport live lives that rely heavily on oil. Almost everything we buy has been produced by a process that consumes oil - it all contains what might be called 'virtual oil'.
Think firstly about the food we eat. Diets in the developed world rely on the cheap transport we get from oil. This applies whether we are eating intensively reared meat flown from the other side of the world or 'organically' grown vegetables driven from 30 kilometres away. Clearly there can be vast differences in the amount of oil consumed in bringing different foodstuffs to us, depending both on the distance travelled and the type of transport used.
In the past, campaigners in the UK have used the concept of 'food miles' to try to capture the differences in the quantities of oil consumed to get different goods to us - their focus was on making consumers aware of the greenhouse gas emissions involved (see Section 7 for more on this). The 'food miles' associated with an item of food measures the distance from the point at which the food was produced to the point at which it is consumed, but the concept has since come under criticism as too simplistic.
For UK 'environmentally engaged' consumers shopping in spring, tomatoes grown in southern Spain might be preferable to home-grown tomatoes - despite the fact that the Spanish tomatoes have many more food miles attached to them. Why might this be true?
The UK-grown tomatoes would have to be grown in heated glasshouses, which could easily involve the consumption of more energy (and the emission of more greenhouse gases) than that required to transport tomatoes from Spain, where they could be grown outdoors or in unheated glasshouses.
Whilst the greenhouse gas emissions resulting from transport are an important part of the environmental impact of the things we buy, it's important to take into account the emissions involved in every stage of an object's production and use. In more recent years, campaigners have tended to use the concept of a 'carbon footprint' - an estimate of the total impact a product has on anthropogenic global warming. It is an area where there are often no clear-cut answers, and differing estimates of environmental impact can often lead to controversy.
The use of oil-based transport makes an almost invisible contribution to the manufacture of all modern goods. I own a cotton jacket (Figure 3) whose label tells me that it was manufactured in Romania. It is immediately apparent that oil will have been used to transport the jacket from Romania to the shop in Manchester where I purchased it (there were probably a few stops along the way). Cotton is not a crop that is grown commercially in Romania, so the cotton will have been imported into Romania from another country - perhaps Egypt or China. This too will have involved the consumption of oil.

(a) A gentleman wearing a white shirt, dark trousers and a sand coloured jacket.(b) A fabric label from the jacket, with the following wording - Made in Romania. Then the size, together with various manufacturers numbers and symbols to indicate how the jacket should be laundered. The composition is given as 100% cotton, body lining 65% viscose, 35% polyester, sleeve lining 100% polyester.
The deceptively simple jacket is an amalgam of different parts manufactured all over the world. It has a lining made from polyester and viscose - two different synthetic materials which are likely to have been manufactured at different sites. It has plastic buttons and is held together by cotton threads. The cloth has been dyed an attractive olive colour - the dye will have been manufactured at one site and transported some distance to the factory that dyed the cloth.
The manufacture of the jacket is only possible because cheap transport fuelled by oil allows the manufacturer to assemble all the parts needed. This relatively complex process of bringing together parts from all over the world applies to almost all the goods around us in our homes. You have explored the manufacture of a jacket - but think of how many more parts and materials have to be produced separately and brought together to manufacture a complex object like a television or computer.
It is very difficult to state accurately how much of an individual's daily crude oil consumption is taken up by the 'virtual oil' in the goods they use. However, you can make a rough estimate. According to International Energy Authority statistics, approximately 61% of crude oil consumed is used for transport (this includes both personal transport and the transport of goods), and you have estimated that on average each individual in the UK is responsible for the consumption of 4 litres of crude oil.
Use these figures to estimate the total daily volume of crude oil that, on average, is used for transport for each individual in the UK.
If 61% of crude oil is used internationally for transport, you can estimate that each individual in the UK consumes an average of approximately 4 litres × (61/100) = 2.4 litres per person for transport alone.
This estimate of a daily consumption of 2.4 litres of crude oil for transport includes both the personal and public transport discussed in Section 2 and the 'virtual' crude oil in the goods and food used by each individual.
Most crude oil is ultimately used as fuel - so while in the UK a person might on average use 2.4 litres of their 4 litre allocation for transport in all its forms, a further 1.1 litres are used as fuel to provide energy for activities other than transport - this includes domestic heating fuel, and fuels for industrial and agricultural processes.
4 Oil as a source of modern materials
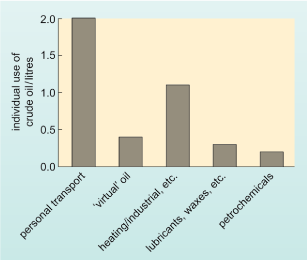
A bar chart with the vertical axis labelled individual use of crude oil/litres and with values from zero to 2.0 litres marked in intervals of 0.5 litres. There are 5 bars. Working from left to right, the labels and heights of the bars are as follows: personal transport 2.0 litres; 'virtual' oil about 0.4 litres; heating/industrial, etc. 1.1 litres; lubricants, waxes, etc. 0.3 litres; petrochemicals 0.2 litres.
So far, 3.5 litres of the UK citizen's 4 litre daily allocation of crude oil have been accounted for. The bulk of the remaining 0.5 litres is used as lubricants, waxes and road coverings, but a small proportion of the allocation - in the region of 0.1−0.2 litres - is converted to petrochemicals (Figure 4). As the name implies, these are chemicals derived from crude oil (petroleum), although the term is also used to refer to products derived from natural gas. Petrochemicals are used to manufacture a huge variety of materials - including plastics, pesticides, drugs, adhesives, paints, and so on.
The proportion of crude oil used to make petrochemicals is small but it is a very important part of the story. The economic importance of petrochemicals can be seen from the fact that recently the world production of transport fuels from crude oil was estimated to have a value of US $385 billion, whilst world production of petrochemicals was estimated to have a value of US $375 billion. Over 80% of crude oil is used to make transport fuel, while 3-5% is used for petrochemicals, yet their value is roughly the same.
Examining the statistics is one way to gain an insight into the importance of petrochemicals, but another way is to look at the things around you in your home. Let's return momentarily to my jacket: the lining is made of the synthetic materials polyester and viscose (Figure 3b) - products that are both to a greater or lesser extent derived from crude oil. More precisely, these materials are made from petrochemicals.
Polyester is ultimately derived from a petrochemical called xylene. Viscose is derived from cellulose extracted from plants, in a manufacturing process that incorporates a number of petrochemicals. The plastic buttons, and the dye too, are almost certainly derived from petrochemicals.

A photograph of various items including clothes, candles, adhesive tape, face and hand creams, chewing gum, washing line, string, tin of paint, aerosol can of insect repellent, baby oil, disinfectant, washing powder, fabric dye, mothballs, a can of butane, a petrol can and a pair of trainers.
It's hard to exaggerate how commonplace petrochemical products have become in our daily lives - we are surrounded by them (Figure 5). We walk on petrochemical products in the form of synthetic fibre carpets and vinyl floorings. The colours on the walls which surround us are derived from petrochemicals. We sit on seats stuffed with plastic foams derived from petrochemicals. Crude oil is the source of almost all the many plastics we use. For example, we get clean water from plastic pipes, whilst other plastic pipes take our waste water away. Our music players, computers and mobile phones are all made in part from plastics derived from oil. The food we eat doesn't just rely on oil for the transport elements of its production; it also relies on the pesticides, herbicides and fertilisers which are usually chemically derived from oil. The packaging which keeps our food fresh or allows us to transfer it directly from the fridge to the oven is derived from oil. Modern medicines are almost always derived from oil, as are replacement heart valves and artificial limbs.
The list of modern goods that rely on the products of the petrochemical industry is almost endless - that is why the small fraction of a barrel of oil used for petrochemicals is so valuable.
5 Is the oil running out?
We usually find oil in new places with old ideas. Sometimes, also, we find oil in an old place with a new idea, but we seldom find oil in an old place with an old idea. Several times in the past we thought we were running out of oil whereas we were only running out of ideas.
We are now entering the second half of the Oil Age, and face the relentless decline of production, imposed by nature.
Oil is one of a number of fossil fuels - fuels derived from the fossilised remains of living things. The two other most abundant fossil fuels are natural gas and coal.
The supplies of all fossil fuels are finite and (in theory) if we keep using them, they will eventually run out. The imminent exhaustion of oil supplies has been predicted a number of times in the last 100 years. In 1919 the head of the prestigious US Geological Survey predicted that the United States would run out of oil within nine years. Almost 100 years later, oil is still being extracted in the US. Discoveries of new oilfields and more efficient extraction techniques have so far kept the oil flowing, though the quantity of oil produced has declined.
There is no question that at some point the oil will run out, but this is unlikely to happen in our lifetimes or even our children's lifetimes. However, when the oil wells run dry is not the key question - or at least not the one likely to affect our or the next generation. Instead, the key question is whether we can produce enough oil to satisfy global demand. Consider Figure 6, produced in 2003 by members of the campaigning organisation the Association for the Study of Peak Oil. The figure shows an estimate of the global production of oil over a 120-year period.
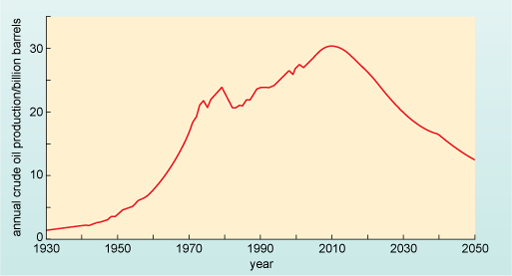
A graph with the vertical axis labelled annual crude oil production/billion barrels and running from 0 to 35, in intervals of 5. The horizontal axis runs from1930 to 2050, in intervals of 10 years. The curve runs gently upwards from a value of about 1 billion barrels in 1930 to 3 billion barrels in 1950, then rises more steeply to about 20 million barrels in 1970. After a peak around 1979 at 22 million barrels, the curve falls back to 20 million barrels in 1981 and then resumes its upward trend to reach a rounded peak at about 30 billion barrels around 2010. After that, the curve falls steadily to about 13 billion barrels in 2050.
Describe the overall shape of the graph in Figure 6.
The graph shows global production of oil rising to a peak at around 2010 and thereafter the production seems set to decline. The peak oil production is approximately 30 billion barrels per year.
Question 3
(a) Using information from Section 1 and Figure 6, calculate the oil production (in tonnes) for 2010, assuming production reached the peak predicted by the Association for the Study of Peak Oil.
(b) Now use Figure 7 to estimate the demand for oil in 2010. According to this data, does supply exceed or meet demand? Comment on the likely future trend in supply and demand for crude oil, if this data is correct.
Answer
(a) According to Figure 6, the production of oil peaked at around 30 billion barrels (30 000 million barrels) in 2010. This can be converted into millions of tonnes of oil as follows:
peak annual oil production in million tonnes
= 30 000 million barrels × 0.14 tonnes per barrel
= 4200 million tonnes per year
(b) From Figure 7, in 2010 the demand for crude oil can be estimated as approximately 4400 million tonnes. So if this data is correct, demand for oil is approximately balanced by its production; but according to the two graphs, demand may very soon exceed supply.
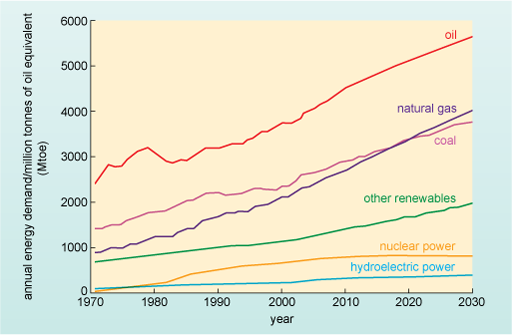
A graph showing the consumption of various energy resources with respect to time. The vertical axis is in Mtoe (millions of tonnes of oil equivalents) from 0 to 7000 in intervals of 1000. The horizontal axis is labelled over 60 years from 1970 to 2030 in decade intervals. The first curve on the graph represents the increase in oil usage over the 60 year period as it rises from 2500 Mtoe to 5750 Mtoe. The next two curves represent natural gas and coal. These rise less steeply over the 60 year period. Natural gas rises from about 1000 Mtoe to 4000 Mtoe and coal from 1400 Mtoe to 3500 Mtoe. The next curve is energy from other renewable resources which rises slowly from 750 Mtoe to 1800 Mtoe over 60 years. The two final curves show the rise in nuclear and hydro power. These two curves show little increase over the 60 years both starting from about 10 Mtoe increasing to 800 Mtoe for nuclear power and 400 Mtoe fro hydro power. In general the graph predicts that oil and other fossil fuels will still be the major sources for world energy consumption towards the year 2030, with oil being consumed the most.
The idea that we are already at, or about to hit, 'peak oil' is a highly disputed one - and heated arguments have taken place about the validity of the ideas behind Figure 6. The debate can take at least two forms. Put crudely, the first form of the debate is between the geologists and the economists. Geologists point to the declining rate of discovery of new oilfields and the inevitable decline in resources. Economists claim that shortages of oil will raise prices - spurring technological development and the exploitation of sources of oil previously considered uneconomic. A second form of the debate occurs between different geologists - over exactly when the peak in oil production is likely to occur.
6 Future sources of oil
The debate as to when, or if, 'peak oil' will occur, centres in part on the extent to which it will be possible to exploit new sources of oil. There are many in the oil industry who argue that the new, so-called 'non-conventional' sources of oil will become far more important in the future.
These new sources of oil are all more expensive than conventional oil, and their exploitation will pose questions about what environmental consequences we are prepared to accept in order to satisfy our oil habit. You will look at each of these non-conventional sources of oil in turn, in order to assess their potential to fill the gap between demand and supply of oil, and to assess their possible environmental impacts. Firstly, you need to know how 'conventional' oil is extracted.
6.1 Conventional oil
Conventional oil is a loosely defined term covering any crude oil that can be extracted from the ground in the 'conventional' way; that is, it is extracted using standard oil well technology.
Conventional oil is generally found in underground rocks known as reservoirs, where it can sometimes be associated with natural gas. The oil is generally under great pressure and initially when an oil well is drilled, the oil will spontaneously flow to the surface. Indeed the problem at this stage is how to control the flow and avoid a 'blowout', an occurrence that plagued the early oil wells and cost many lives (Figure 8).

A black and white picture of a drilling rig with a plume of black oil shooting vertically upwards out of the picture, with some of it beginning to fall back down.
Modern oil wells are designed to avoid disastrous blowouts and have sophisticated pressure release systems which allow the controlled release of oil at the surface. However, blowouts still occur occasionally, as you will see in Section 6.2.
The stage at which oil flows spontaneously to the surface is known as the primary recovery phase (Figure 9a) and only a small proportion of the oil in the reservoir can be extracted in this way. Eventually, the pressure in the oil reservoir will drop, and to prolong extraction it becomes necessary to maintain the pressure underground by injecting pressurised water or gas, or both, into the reservoir (Figure 9b). This phase of production is known as the secondary recovery phase.
In order to improve recovery still further, chemical or biological agents may be added to the injected water, or steam may be pumped into the reservoir, in order to reduce the viscosity (thickness) of the crude oil. The term 'viscosity' may be new to you; in scientific terms, viscosity is a measure of how easily a liquid flows - a liquid with low viscosity such as water flows easily, whilst a liquid with high viscosity (e.g. treacle or honey) does not flow easily.
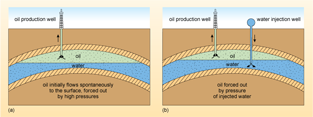
These are two similar sketches of vertical sections through the Earth's surface. They show a gentle upwards fold of three rock strata below the surface. There are narrow bands of impermeable rock top and bottom and a central larger section of permeable rock. The permeable rock has oil filling the upper part, floating on water below, with a horizontal line separating them. In (a), there is an drilling rig with a pipe down into the oil layer and arrows showing that the oil flows under its own pressure into the pipe and upwards to the surface. In (b), in addition to the oil production well seen in (a), there is also a water tank at the surface with a pipe down into the water layer, labelled water injection well. There are arrows to show that the water is pumped down the pipe and out into the water layer below the oil. This results in the water level below the oil being higher in (b) than in (a). Oil is then forced up into the pipe of the oil production well and up to the surface in the same way as in (a).
Under ideal conditions, secondary recovery methods can result in up to 70% of the initial oil present in the reservoir being recovered, but the processes are expensive and for many smaller fields the amount of extra oil recovered may not be worth the investment.
6.2 Non-conventional oil
It is generally agreed that there are huge quantities of non-conventional oil still to be extracted, and the quantities are estimated to be greater in total than those of the unextracted conventional oil remaining. However, by definition the non-conventional oil is much harder to get out of the ground and to process. The two types of non-conventional oil most often discussed are oil sands and heavy oils, and you will briefly explore issues related to these. You will also look at three other types of oil that might be classed as non-conventional: deep-water oil, polar oil and natural gas liquids.
Oil sands

An aerial photograph of a piece of land, with what appear to be brownish tracks criss-crossing it, and paler brown areas between. At the top of the picture, the land appears white as if covered by snow.
Oil sands (also known as tar sands) are formed when oil flows into near-surface sands from deeper underground reservoirs. Exposure of crude oil to air and bacteria close to the surface degrades the oil into thick, viscous bitumen, with the consistency of very thick treacle. The fact that the bitumen is mixed with sands, clays and water - together with its extreme viscosity - makes it difficult and expensive to extract the oil. Until very recently, oil sands have been collected by large-scale strip mining (Figure 10), which involves scooping up vast quantities of the sands using huge machines. The enormous volumes of oil sands are then heated to around 80 °C to separate and chemically change the bitumen to produce a material that flows more easily and has a chemical make-up more similar to that of conventional crude oil. This is a complex, energy-intensive and expensive process, but in recent years, as the price of oil has increased, it has become an increasingly competitive alternative to conventional oil sources.
Where oil sands lie too far beneath the surface to allow surface mining, huge volumes of steam or hot carbon dioxide gas are injected deep below ground; after several months, the viscosity of the oil is lowered enough to allow the oil to be pumped to the surface.
The world's largest producer of crude from oil sands is Canada, where the Athabasca area of northern Alberta has reserves of oil second only to those of Saudi Arabia. It takes two tonnes of oil sands to produce one barrel of oil.
What percentage by mass of oil sand is recovered as crude oil, and what does this tell you about the amount of waste generated by this process?
One barrel of oil has a mass of approximately 0.14 tonnes, so two tonnes of oil sand might produce approximately 0.14 tonnes oil.
So percentage of oil recovered = (0.14 tonnes/2 tonnes) × 100% = 7%
This implies that a staggering 93% of the oil sand is discarded as waste.
The oil from oil sands has been described as the dirtiest oil on Earth. The huge-scale strip mining destroys habitats (Figure 10). The processing creates huge quantities of toxic residues. It takes three to five times more energy to extract crude oil from oil sands than it takes to extract conventional oil. According to one estimate, this means that burning a litre of petrol from oil sands results in 20% more greenhouse gas emissions than burning a litre from conventional sources.
Heavy oils
Heavy oils are highly viscous deposits of crude oil which will not flow to the surface under their own natural pressure. The largest resources are the Orinoco heavy oils of Venezuela, which represent almost 90% of the known reserves of this form of crude oil. Like oil sands they have to be specially treated before they can be used in conventional oil refineries. Heavy oils tend to have a composition that makes them less economic to process once they have been extracted: for instance, they contain relatively high levels of sulfur and heavy metals, which have to be removed before normal processing.
Heavy oils suffer many of the same environmental problems as oil sands. The extraction is very energy-intensive, resulting in higher greenhouse gas emissions than those for conventional oil, and the presence of contaminants can lead to large volumes of toxic wastes.
Deep-water oil
As the price of oil has climbed, companies have found it profitable to explore for and extract oil from less accessible places. In the context of undersea oilfields, this has led to a trend towards the exploitation of resources found in increasingly deeper water. For environmentalists, the dangers of this trend were exposed by the Deepwater Horizon oil spill in April 2010. The pressure control systems of the oil well failed, leading to a huge underwater blowout. The explosion killed 11 workers on the drilling rig and an estimated 2.9 million barrels of oil flowed into the waters of the Gulf of Mexico. The full ecological consequences of the spill have, at the time of writing (2011), yet to be determined.
Polar oil
The Arctic is seen by many as an unspoiled wilderness that should be protected - iconic images of polar bears trapped on melting ice floes have often been used to dramatise the effects of anthropogenic global warming. But many of the states bordering on the Arctic view the region in quite a different way - they see it as a potential source of valuable oil and minerals. The Arctic is estimated to hold 13% of the world's resources of oil (and 30% of its gas). The US Geological Survey has suggested that it is the largest unexploited source of conventional oil in the world.

A photograph at sea with an ocean-going tug in the foreground, completely dwarfed by a huge floating 'factory' with a drilling rig, a huge chimney, several cranes and a helicopter landing deck.
Drilling for oil in the Arctic poses a number of problems: the extremely low temperatures, the perpetual night in winter, shifting pack ice, icebergs and storms. So far, oil companies have concentrated their efforts around the more accessible fringes of the Arctic. The next phase of oil recovery is likely to focus on sheltered shallow-water regions close to shore. However, plans are well under way to start exploring for oil in the less accessible parts of the area. In order to drill in deeper water, vast steel platforms are being built, such as the Russian Prirazlomnaya platform (Figure 11). The huge bulk of such platforms will stop them from being crushed by the shifting pack ice that covers the sea for eight months of the year. However, these platforms will not be suitable for drilling in really deep Arctic water where it's likely that ice-resistant ships will float on the surface collecting oil from underwater wells below.
Plans to rapidly develop Arctic oil resources have enraged environmental activists, who question the safety of deep-water drilling - especially in the light of the Deepwater Horizon incident. Others argue that the real risk will not be from the wells themselves but from the oil tankers which will have to make their way through the icebergs and pack ice. Oil companies are researching the best ways to avoid oil spills and to mop them up if they do happen. But the environmentalists are not convinced, and many have called for a moratorium on all Arctic oil exploration. This can set them against the local population who see the oil industry as a vital potential source of income.
Natural gas liquids
When natural gas is extracted from the ground it is often associated with a variety of liquids. These are separated from the gas and can be used to substitute for certain products from crude oil. As production of natural gas grows over the next few decades, it is likely that the use of natural gas liquids will become increasingly important; but it is unlikely that the level of production will replace more than a minor proportion of the oil we use.
The extraction of oil from ever more difficult sources has environmental consequences that some see as unacceptable; whilst others argue that continued extraction of oil is necessary to maintain living standards in the developed world and allow their improvement in the developing world. But there is one vitally important aspect of the environmental impact of oil only touched on so far - its contribution to anthropogenic global warming.
7 Oil and anthropogenic global warming
Since the mid-19th century, human industrial and agricultural activity has caused a change in the concentrations of gases in the Earth's atmosphere. In particular, there has been a dramatic increase in the concentration of atmospheric carbon dioxide (Box 1), caused primarily by the combustion of fossil fuels.
Box 1 Carbon dioxide and chemical formulas
Carbon dioxide is a colourless, non-toxic gas which occurs naturally in the atmosphere. It is produced whenever compounds containing carbon are burnt in air, and is also produced as a result of respiration - the process by which organisms (including humans) convert their food into energy. Carbon dioxide has the chemical formula CO2. This means that carbon dioxide gas is made of tiny units each comprising one atom of carbon and two atoms of oxygen. These units are called molecules. (The subscript '1' is assumed if no other number is written.) It's important to note the way the chemical formula is written: capital C (for carbon), capital O (for oxygen) followed by a subscript 2, i.e. it is CO2 not CO2 or co2.
Figure 12 shows the emissions of carbon dioxide resulting from combustion of fossil fuels (and cement production) over the last 200 years.
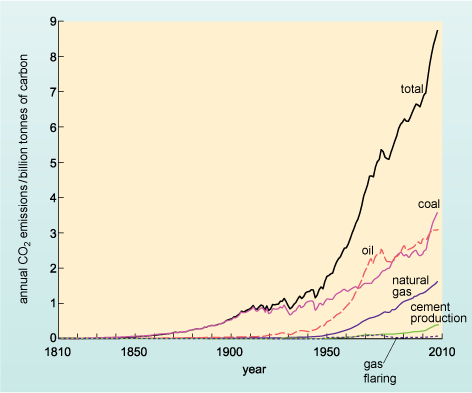
A graph with the horizontal axis marked in years from 1810 to 2010, in intervals of 10 years. The vertical axis is labelled annual CO2 emissions/billion tonnes of carbon and runs from 0 to 9 in intervals of 1. There are 6 lines on the graph, labelled gas flaring, cement production, natural gas, oil, coal and total. The gas flaring line runs almost along the horizontal axis for the whole of its length, with a very slight rise in the 1970s to less than 0.1 billion tonnes of carbon emitted, then a fall again, and a continuing very slight rise after 2000. The cement production line also runs along the horizontal axis for most of its length, then starts to creep upwards very slightly from about 1950 onwards and rises very gently to about 0.3 billion tonnes of carbon emitted in 2008. The natural gas line runs along the horizontal axis till about 1940 and then rises, very slowly at first, then slightly faster to reach its highest level at about 1.5 billion tonnes of carbon emitted in 2008. The line for oil leaves the horizontal axis in about 1910 and rises slowly at first, then much faster from 1940 onwards to reach a peak in about 1980 of 2.5 billion tonnes of carbon emitted. There is then a slight fall and the rise resumes to reach just over 3 billion tonnes in 2008. From about 1840 onwards, the line for coal rises slightly irregularly, but generally steadily upwards to about 2000 when about 2.3 billion tonnes of carbon are emitted, then there is a very steep rise over the next 8 years to about 3.7 billion tonnes. The final line, labelled total, is the sum of all the other lines. From about 1840 onwards, since only the emissions for coal are significant, it rises with the coal line, but then gradually rises above it, as the other sources add in. By 1950, the total is about 1.8 billion tonnes and then the line rises very steeply, though in a slightly jagged way, to nearly 9 billion tonnes in 2008.
Examine Figure 12. Until the 1950s, which fuel contributed most to anthropogenic carbon dioxide emissions?
Coal.
Approximately when were the emissions from this fuel first overtaken by those of oil?
In about 1970.
At present the combustion of coal and oil are responsible for broadly similar levels of CO2 emissions, whilst emissions from the combustion of natural gas are approximately half those of either coal or oil.
Natural gas consists primarily of methane, a substance made up of carbon and hydrogen. Given that each molecule of methane contains one atom of carbon and four atoms of hydrogen (symbol H), what is the correct way to write the chemical formula of methane?
CH4 - capital C (carbon), capital H (hydrogen), with subscript 4. If you wrote H4C you are not, strictly speaking, wrong but chemists always tend to put carbon at the beginning of a chemical formula.
In order to more fully describe the combustion of coal, natural gas and oil, it is necessary to use chemical equations, as described in Box 2. If you have studied chemistry recently, the concepts may be relatively familiar and you may be able to read through this box quickly, or skip it completely.
Box 2 Introducing chemical equations
When chemists want to describe a chemical reaction in which one set of substances (the reactants) are transformed into another (the products), they usually do so by means of a chemical equation. The reactants are shown on the left with an arrow leading to the products on the right:
Coal is made up mostly of carbon atoms (C). Oxygen in the air is made up of pairs of oxygen atoms, written as O2. When coal burns, the carbon and oxygen atoms combine to make carbon dioxide. The ash left after a coal fire is largely the part of the coal that was not carbon and so did not burn away to carbon dioxide.
The combustion reaction between carbon and oxygen would be written like this:
Notice that there must be the same amount of carbon and the same amount of oxygen (measured here by the number of atoms) on each side, since you cannot create more carbon or oxygen, or lose any, in a chemical process. This applies to all chemical equations like this. They must be 'balanced' with the same number of atoms on each side. It is also useful to indicate whether the substances taking part in the reaction are solids, liquids or gases, so the letter s, l or g is placed in brackets after each substance. So, since the coal is a solid, and the oxygen and carbon dioxide are gases, the complete version of reaction 1.1 would be:
Now consider what happens when natural gas, methane CH4, is burnt in a similar way. This time the products include water, whose chemical formula is H2O.
Try writing the equation in which methane burns in oxygen to produce carbon dioxide and water, in the form of steam. Remember to include the symbols that show whether the substances taking part are solids, liquids or gases.
The equation is:
CH4(g) + O2(g) → CO2(g) + H2O(g)
The numbers of atoms on each side are not the same; there are three oxygen atoms on the right-hand side, but only two on the left. Since the oxygen atoms are in pairs on the left, there needs to be an even number on the right too. That can be done if the reaction produces two molecules of water. So the next stage is to write:
CH4(g) + O2(g) → CO2(g) + 2H2O(g)
Now, counting the carbon atoms shows that there is one on each side, and there are four hydrogen atoms on each side (four in the methane on the left, and two water molecules, each with two hydrogen atoms, so that makes four on the right too). However, there are only two oxygen atoms on the left and four on the right (two in CO2 and one in each of the two water molecules). But if you have two molecules of oxygen on the left, then that gives four oxygen atoms on both sides too. The final equation for the combustion of methane can be written:
An equation like this, with equal numbers of atoms on both sides, is said to be balanced, and often a fully balanced equation will be written with an equals sign (=) to replace the arrow (→). So the equation can also be written as:
A chemical equation written with an 'equals' sign emphasises that it is balanced, and this form is often used in Open University texts.
Question 4
Propane is a gas derived from the processing of crude oil. It is used as a fuel for heating and cooking. Propane consists of molecules containing three atoms of carbon and eight atoms of hydrogen. Give the chemical formula of propane and write an unbalanced chemical equation to show its combustion.
Answer
The chemical formula of propane is C3H8 - it is very important that the letters C and H are capitals, and that the numbers are subscripts.
An unbalanced chemical equation for the combustion of propane is:
Note that the unbalanced equation uses an arrow. The full balanced equation, which you were not asked to give, can be written:
Once again the full balanced equation can be written using an 'equals' sign (=) rather than an arrow (→).
The fossil fuels burned in the 200 years since the Industrial Revolution were formed underground over many millions of years. The sudden return of ancient carbon to the atmosphere upsets the outer Earth's energy balance, and is responsible for anthropogenic global warming. This is an issue of much concern among scientists, environmentalists and politicians.
The science behind global warming will not be discussed in any detail in this course; but the following text will discuss briefly why gases like carbon dioxide affect the global temperatures. A number of gases like CO2 absorb some of the Sun's energy which might otherwise be reflected back into space, resulting in an overall warming of the atmosphere. Gases that can act in this way are called greenhouses gases. The two major greenhouse gases are water vapour (H2O) and carbon dioxide (CO2), although methane (CH4) is also an important greenhouse gas. Without the warming effect of these naturally occurring gases, the temperature at the Earth's surface would be closer to −20 °C than the current mean value of 15 °C. Increases in carbon dioxide and other greenhouse gas concentrations will inevitably lead to increases in global mean surface temperatures.
The increasing concentration of CO2 in the atmosphere has already resulted in small but measurable increases in global temperatures. In fact, the 20th century saw an increase in the annual mean surface temperature of about 0.7 °C. Predicting future global warming is beset with uncertainties, but our best estimates are that if there is no change in the current 'blend' of energy sources (known as the 'business-as-usual' scenario), the global mean surface temperature will rise by between 1.1 °C and 6.4 °C over the next 100 years or so.
Increased surface temperatures are likely to have a variety of effects. One major effect would be on sea levels: increases in global temperature are likely to be accompanied by a rise in global sea level, because of thermal expansion of the oceans and melting of land-based ice. A 'business-as-usual' scenario suggests a sea-level rise of between 0.2 m and 0.65 m by the end of the 21st century. Low-lying island states are particularly threatened by rising sea levels; but other effects could include greater incidence of storm-surge flooding, higher coastal erosion, and loss of property and coastal habitats. Rising sea levels could have devastating social and economic consequences.
Other effects of anthropogenic global warming include changes to the timings of the seasons, with effects on agriculture, changing distribution of diseases and damage to ecosystems.
To stabilise carbon dioxide levels would require an immediate reduction of emissions from human activities. Yet even if fossil fuel burning stopped immediately, the temperature rise would continue because of the time lags in atmospheric systems.
The combustion of oil is a major contributor to carbon dioxide emissions - contributing just over one-third of all anthropogenic emissions (Figure 12), a proportion similar to the emissions produced from coal, which is primarily burnt to generate electricity.
Clearly if we are to reduce emissions of carbon dioxide, reducing our reliance on oil will not by itself be enough - we definitely need to do something to replace our coal-fired power stations. Nonetheless, developing sustainable, efficient alternatives to oil-based fuels could make a major contribution to efforts to reduce greenhouse gas emissions.
8 Other pollution from conventional road transport
Recent concerns in this area have tended to focus on the problem of carbon dioxide emissions, but our current modes of road transport are also responsible for the emission of various other pollutants. You will briefly explore these here, as any alternative to oil-based road transport should address the need to minimise these pollutants as well as reduce carbon dioxide emissions.
Carbon monoxide
When any carbon-based fuel is burnt, carbon monoxide (CO) can be formed if insufficient oxygen is present.
Write out the equation in which methane gas (CH4) is burnt in oxygen gas (O2) to produce carbon monoxide gas (CO) and gaseous water (H2O).
The equation is:
CH4(g) + O2(g) → CO(g) + H2O(g)
The balanced version of this equation can be written:
Check for yourself that all the atoms on the left of Equation 4 appear on the right, and none have been lost.
Compare Equation 4 with Equation 3 in which methane burned to produce carbon dioxide. How does the amount of oxygen used in each reaction compare?
When methane is burned to produce carbon dioxide, it uses twice as many molecules of oxygen as there are of methane. When methane burns to produce carbon monoxide, it only uses 1½ times as many oxygen molecules as methane molecules (three oxygen molecules to every two methane molecules).
Carbon monoxide pollution can be a major problem in urban areas; it contributes to the formation of smog and is highly toxic - it combines with haemoglobin in the blood, which hinders the body's ability to take up oxygen. This can cause and aggravate respiratory and heart disease.
Nitrogen oxides
At the high temperatures generated in internal combustion engines, the nitrogen in air (N2) can combine with oxygen to form various nitrogen oxides - most importantly, nitrogen monoxide (NO) and nitrogen dioxide (NO2).
Write equations to show how nitrogen can combine with oxygen from the air to form both of these nitrogen oxides. You should be able to balance the equations.
To form nitrogen monoxide, NO, the equation would be:
N2(g) + O2(g) → NO(g)
and the balanced version can be written:
N2(g) + O2(g) = 2NO(g)
To form nitrogen dioxide, NO2, the equation would be:
N2(g) + O2(g) → NO2(g)
and the balanced version can be written:
N2(g) + 2O2(g) = 2NO2(g)
Together nitrogen monoxide and nitrogen dioxide are referred to as NOx. Collectively, nitrogen oxides are toxic, lead to the formation of severe acid rain, are greenhouse gases in their own right and are responsible for the formation of tropospheric ozone (see below).
Particulates
Diesel engines emit tiny dust-like particles of soot and other substances, known collectively as particulates, which are responsible for respiratory problems and thought to be carcinogenic (cancer-causing). They also contribute to smog formation.
Volatile organic compounds (VOCs)
These are carbon-based (organic) compounds that evaporate easily into the air - they are volatile. Various VOCs are emitted as a result of using diesel and petrol fuels. Many of them are carcinogenic and because they are in the air, they are easily inhaled and can cause damage to the lungs.
Tropospheric ozone
In the upper atmosphere or stratosphere, ozone - a form of oxygen with three oxygen atoms in its molecule (O3) - is constantly being formed and broken down. It is beneficial to life on Earth since it absorbs ultraviolet (UV) light, so reducing the number of harmful UV rays reaching living organisms on the Earth's surface. However, near ground level (in the troposphere), ozone is toxic and responsible for aggravating respiratory problems in humans and reducing crop yields. Tropospheric ozone is not produced directly by internal combustion engines, but from a complex series of chemical reactions involving VOCs, nitrogen oxides and carbon monoxide. Its presence contributes to smog formation.
Question 5
Complete Table 2 which summarises the impacts of pollutants emitted as a result of combustion of oil-based road fuels. Insert chemical formulas, 'yes' or 'no', as appropriate.
| Pollutants | Chemical formula | Toxic? | Contributes to anthropogenic global warming? | Contributes to formation of severe acid rain? | Causes smog formation? |
|---|---|---|---|---|---|
| carbon dioxide | no | yes | no | no | |
| carbon monoxide | no | no | |||
| nitrogen oxides | NOx | ||||
| particulates | not applicable | unclear | no | ||
| volatile organic compounds (VOCs) | not applicable | no | |||
| tropospheric ozone | no |
Answer
See Table 3.
| Pollutants | Chemical formula | Toxic? | Contributes to anthropogenic global warming? | Contributes to formation of severe acid rain? | Causes smog formation? |
|---|---|---|---|---|---|
| carbon dioxide | CO2 | no | yes | no | no |
| carbon monoxide | CO | yes | no | no | yes |
| nitrogen oxides | NOx | yes | yes | yes | yes |
| particulates | not applicable | yes | unclear | no | yes |
| volatile organic compounds (VOCs) | not applicable | yes | yes | no | yes |
| tropospheric ozone | O3 | yes | yes | no | yes |
9 Conclusion
Many environmentalists argue that we should be attempting to change individuals' behaviour rather than increase the efficiency of transport technologies. This implies a dramatic reduction in the use of the most energy-intensive modes of transport - car and air travel. But many individuals and politicians baulk at the prospect of 'turning the clock back' to a level of mobility considerably less than that we currently enjoy. Car use is now deeply entrenched in our society and economy, however environmentally problematic that may be.
There are clearly two extreme views here. One might be that we can solve the issue of anthropogenic global warming if we get the technology right. The other extreme would be to argue that only by changing our transport habits can we hope to begin to reduce emissions. You will have to decide where you stand on this issue. Perhaps, like the author, you will feel that the truth lies somewhere between these two extremes.
Activity 2 The peak oil debate
The estimated time for this activity is 40 minutes.
While studying this course you have explored some important trends in oil production and consumption in the United States, China and the Middle East. For many commentators, these trends are not as important as the bigger question: is overall global oil production about to decline? Many argue that we are at, or very close to, the point when the world's production of oil will peak and start to fall (Section 5). This idea, known as the 'Peak Oil hypothesis', is a controversial one and in this activity you will explore the views of two commentators from opposite sides of the debate.
You will listen to short extracts from interviews with the two. You should bear in mind that these are extracts rather than the full interviews.
If you have time, you may wish to listen to the whole of each extract through once, and then read the questions before listening to the extract again. Use the pause button while noting down your answers to each question.
Part I
The first commentator is Dieter Helm, Professor of Energy Policy at the University of Oxford, who specialises in issues related to energy and the environment. He doubts the validity of what he calls 'Peak Oil' hypotheses.
Listen to the 5-minute extract of the interview and then answer the following questions.

Transcript: Audio 1
Question
- a.Dieter Helm outlines three related, competing Peak Oil hypotheses. What are they and what do they have in common?
- b.He argues that it is not the supply of oil that is limited but our technologies. Why does he feel that technology will improve?
- c.What are the two key ways in which he thinks new technologies can increase supply?
Answer
- a.The three competing Peak Oil hypotheses he describes are:
- the Earth's oil resources are physically limited
- oil that we need to use now is controlled by a small number of countries, which may restrict supplies for political reasons
- inevitably demand for oil will rise so fast that supply will not keep up - making the price of oil soar.
- b.He argues that as the price of oil rises, investment in new technologies becomes economic.
- c.The two key areas in which he thinks technology will improve are:
- getting more out of existing conventional wells, where we currently leave up to 50% of the oil in the ground
- extracting unconventional oils such as shale oils.
Part II
Shaun Chamberlin is an environmental activist, author and policy advisor. He was a pioneer of the Transition Towns movement and sits on the council of the campaigning organisation World Development Movement. He has also served as an advisor to the UK Department of Energy and Climate Change and co-authored the All Party Parliamentary Group on Peak Oil's 2011 report into energy rationing. He argues that Peak Oil is a reality now.
Listen to the 5-minute extract of the interview and then answer the following questions.

Transcript: Audio 2
Question
- a.Dieter Helm outlined three related Peak Oil hypotheses. Which sort of Peak Oil arguments does Shaun Chamberlin put forward and what sort of evidence does he draw on?
- b.Why does he argue that 'It's not the size of the tank that matters, it's the size of the tap'?
Answer
- a.Shaun Chamberlin's arguments largely focus on the physical limitations of oil supply - the evidence he draws on are past levels of oil production.
- b.He feels that Peak Oil issues are related to the rate at which oil is extracted from the ground, rather than how much is stored there. He argues, for instance, that the extraction of oil from oil shales is a very slow process - limiting the rate at which oil can be produced from this source.
If you are interested to read more about Shaun Chamberlin's views, he writes at www.darkoptimism.org.
Part III
Question
Having listened to the two commentators, and read this course, where do you stand on the Peak Oil question? From what you know, do you think it is a real issue? In your opinion does it affect the question of whether or not we should be trying to live without oil? (Answer in about 150 words)
Answer
Your answer here will clearly depend on your point of view. Here is one possible answer, though you may take a different stance.
Supplies of conventional oil are likely to peak in the near future, but if we are prepared to invest in expensive technologies to extract unconventional oil like shale oil, we will be able to keep supplies going for a very long time. This will mean that the prices of oil products are likely to remain high. The extraction of unconventional oil is likely to have environmental consequences, for example the extraction of shale oil is a very dirty process. The question of whether or not oil supplies will peak is not relevant when we consider the issue of anthropogenic global warming - we need to find ways to supply our energy needs without burning fossil fuels. For this reason the question of if we can live without oil and other fossil fuels is important, whether or not supplies are about to decline.
Conclusion
- Oil plays a central role in the modern world.
- The major use of oil is as a cheap source of transport fuel. We rely on cheap transport to move around both goods and people. Cheap oil-driven transport plays an almost invisible role in the manufacture of all modern goods.
- A smaller but vitally important role of oil is as a source of modern materials, particularly plastics.
- The need to develop sustainable alternatives to oil is driven by two major factors. The first is the fact that oil is becoming harder to find and extract and is likely to become more expensive, both economically and environmentally. The second is that the combustion of oil is a major contributor to anthropogenic global warming.
- Alternative sources of crude oil are available, but this non-conventional oil is difficult to obtain and often its extraction can cause greater environmental damage than that of conventional oil.
- Combustion of oil as fuel also gives rise to other forms of pollution: for example, carbon monoxide, nitrogen oxides, particulates, VOCs and tropospheric ozone.
- Chemical compounds are described using chemical formulas. For example, methane has the chemical formula CH4, meaning each molecule of methane consists of one atom of carbon and four atoms of hydrogen.
- Chemical change is described using chemical equations. Full balanced chemical equations include the state (gas, liquid or solid) of the substances involved, and can be indicated by using an 'equals' sign. Unbalanced chemical equations use an arrow.
Acknowledgements
This course was written by John Baxter
This free course is adapted from a former Open University course Living without oil (S176).
Course image: Febe Patty in Flickr made available under Creative Commons Attribution-NonCommercial-ShareAlike 2.0 Licence.
The material acknowledged below is Proprietary, used under Licence and not subject to Creative Commons Licence. See terms and conditions.
Grateful acknowledgement is made to the following:
Figures
Figure 2 (v) Ian Britton/freefoto.com (http://creativecommons.org/ licenses/ by-nc-nd/ 3.0/; Figure 5 © Cordelia Molloy/Science Photo Library; Figure 10: © Getty Images; Figure 11 courtesy of Gazprom.
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
This free course is adapted from a former Open University course called 'Living without oil (S176)'.
Copyright © 2016 The Open University