Eutrophication
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Thursday, 18 April 2024, 11:52 AM
Eutrophication
Introduction
Eutrophication describes the biological effects of an increase in the concentration of nutrients. The collective term ‘nutrients’ refers to those elements that are essential for primary production by plants or other photosynthetic organisms. Eutrophication is most often caused by increases in the availability of nitrogen and phosphorus, commonly present in soil and water in the form of nitrate and phosphate, respectively. However, altered concentrations of any plant nutrient may have a recognizable biological effect. Eutrophication can occur in any aquatic system (freshwater or marine), and the term is also used to describe the process whereby terrestrial vegetation is affected by nutrient-enriched soil water.
This OpenLearn course provides a sample of level 2 study in Environment & Development
Learning outcomes
After studying this course, you should be able to:
describe the principal differences between a eutrophic and an oligotrophic ecosystem
explain the mechanisms by which species diversity is reduced as a result of eutrophication (Questions 2.1 and 2.2)
contrast the anthropogenic sources that supply nitrogen and phosphorus to the wider environment, and describe how these sources can be controlled (Question 3.1)
describe how living organisms can be used as monitors of the trophic status of ecosystems (Question 4.1)
compare the advantages and disadvantages of three different methods for combating anthropogenic eutrophication (Question 4.2).
1 Introduction
1.1 Origin of the term ‘eutrophication’
The levels of nutrients present determine the trophic state of a water body, where trophic means ‘feeding’.
SAQ 1
Give another example of the adjective trophic being used in a scientific context.
Answer
Trophic levels, as applied to a food chain.
The adjective eutrophe (literally ‘well fed’) was first used by the German botanist Weber in 1907, to describe the initially high nutrient conditions that occur in some types of ecosystem at the start of secondary succession. Scientists studying lakes at the beginning of the 20th century identified stages in plant community succession that appeared to be directly related to trophic state or nutrient status. They described a series of stages:
‘oligotrophic — mesotrophic — eutrophic — hypertrophic’
where oligotrophic meant ‘low in nutrients’, mesotrophic ‘with intermediate nutrient concentration’, eutrophic ‘high in nutrients’ and hypertrophic ‘very high in nutrients’. At the time, these definitions were derived from comparative estimates between water bodies with different nutrient status, judged according to their phytoplankton communities. Phytoplankton is a collective term for the free-floating photosynthetic organisms within the water column. It encompasses both algae (from the kingdom Protoctista) and photosynthetic members of the kingdom Bacteria. Thus an oligotrophic lake would have clear water with little phytoplankton, whereas a eutrophic lake would be more turbid and green from dense phytoplankton growth, and a mesotrophic lake would be intermediate between the two. Table 1.1 summarizes some of the general characteristics of oligotrophic and eutrophic lakes. A further definition, dystrophic, describes ‘brown-water lakes’, which have heavily stained water due to large amounts of organic matter usually leached from peat soils. The presence of these organic compounds can reduce the availability of nutrients to organisms, making the water body even less productive than an oligotrophic one.
| Characteristic | Oligotrophic | Eutrophic |
|---|---|---|
| primary production | low | high |
| diversity of primary producers | high species diversity,with low population densities | low species diversity,with high population densities |
| light penetration into water column | high | low |
| toxic blooms | rare | frequent |
| plant nutrient availability | low | high |
| animal production | low | high |
| oxygen status of surfacewater | high | low |
| fish | salmonid fish (e.g.trout, char)often dominant | coarse fish (e.g. perch, roach, carp) often dominant |
SAQ 2
Why is light penetration poor in eutrophic lakes?
Answer
The high density of phytoplankton absorbs light for photosynthesis and prevents it penetrating deeper into the water.
More recently, trophic bands have been defined in relation to levels of nutrients measured by chemical analysis. Table 1.2 shows trophic bands as defined in relation to concentrations of total phosphorus.
| Trophic band | Total phosphorus/mg l-1 |
|---|---|
| dystrophic | |
| oligotrophic | 0.005-0.01 |
| mesotrophic | 0.01-0.03 |
| eutrophic | 0.03-0.1 |
| hypertrophic | >0.1 |
The trophic state of water bodies and rivers varies depending on a number of factors, including position in the landscape and management of surrounding land. In general, upland areas are more likely to have nutrient-poor (oligotrophic) water, characterized by relatively fast-flowing rivers (Figure 1.1) and lakes that have clear water with limited higher plant communities.

By contrast, lowland waters in more fertile river catchments tend to be nutrient-rich (eutrophic), and lakes in lowland areas are more likely to be turbid with lush fringing vegetation. Lowland rivers have slower flow and are likely to be more nutrient rich as a result of soluble compounds having been washed into them. They are likely to have fringing vegetation and some floating and submerged aquatic plants (Figures 1.2 and 1.3). In aquatic systems, the term macrophyte is used to describe any large plant (macro, large; phyte, plant). The term is used to distinguish angiosperms (whether emergent, floating or submerged) from small algae such as diatoms (which are strictly not plants at all, but are often lumped together with plants when considering the productivity of ecosystems).


SAQ 3
What is the process by which nutrient elements are lost from the soil profile by the action of excess rainfall draining through it, which may eventually deliver them to a surface water body?
Answer
Leaching.
The term ‘eutrophication’ came into common usage from the 1940s onwards, when it was realized that, over a period of years, plant nutrients derived from industrial activity and agriculture had caused changes in water quality and the biological character of water bodies. In England and Wales, eutrophication has been a particular concern since the late 1980s, when public awareness of the problem was heightened by widespread toxic blue-green bacterial blooms (commonly, but incorrectly, referred to as algal blooms) in standing and slow-flowing freshwaters. Figure 1.4 shows blue-green bacteria (cyanobacteria) growing at the margins of a lake. Cyanobacteria are not typical bacteria, not only because some of them are photosynthetic, but also because some of them can be multicellular, forming long chains of cells. Nonetheless, cyanobacteria clearly belong to the kingdom Bacteria because of their internal cellular structure.

SAQ 4
Why are cyanobacteria so productive in eutrophic water bodies (Figure 1.4) compared with oligotrophic ones?
Answer
The ready availability of nutrients allows rapid growth. In oligotrophic water the rate of growth is limited by the nutrient supply, but in eutrophic water it is often only the availability of light which regulates primary production.
1.2 Resource availability and species diversity
A wide range of ecosystems has been studied in terms of their species diversity and the availability of resources. Each produces an individual relationship between these two variables, but a common pattern emerges from most of them, especially when plant diversity is being considered. This pattern has been named the humped-back relationship and suggests diversity is greatest at intermediate levels of productivity in many systems (Figure 1.5).
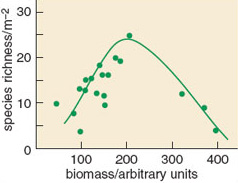
SAQ 5
How does species diversity differ from species richness?
Answer
Species diversity includes a measure of how evenly spread the biomass is between species (equitability) rather than a simple count of the species present.
An explanation for this relationship is that at very low resource availability, and hence ecosystem productivity, only a limited number of species are suitably adapted to survive. As the limiting resource becomes more readily available, then more species are able to grow. However, once resources are readily available, then the more competitive species within a community are able to dominate it and exclude less vigorous species.
In most ecosystems it is the availability of mineral nutrients (especially nitrogen and phosphorus) that limits productivity. In eutrophic environments these nutrients are readily available by definition, so species diversity can be expected to be lower than in a more mesotrophic situation. It is for this reason that eutrophication is regarded as a threat to biodiversity. Eutrophication of the environment by human-mediated processes can have far reaching effects, because the nutrients released are often quite mobile. Together with habitat destruction, it probably represents one of the greatest threats to the sustainability of biodiversity over most of the Earth.
1.3 Natural eutrophication
Eutrophication of habitat can occur without human interference. Nutrient enrichment may affect habitats of any initial trophic state, causing distinctive changes to plant and animal communities. The process of primary succession is normally associated with a gradual eutrophication of a site as nutrients are acquired and stored by vegetation both as living tissue and organic matter in the soil.
There is a long-standing theory that most water bodies go through a gradual process of nutrient enrichment as they age: a process referred to as natural eutrophication. All lakes, ponds and reservoirs have a limited lifespan, varying from a few years for shallow water bodies to millions of years for deep crater lakes created by movements of the Earth’s crust. They fill in gradually with sediment and eventually became shallow enough for plants rooted in the bed sediment to dominate, at which point they develop into a closed swamp or fen and are eventually colonized by terrestrial vegetation (Figures 1.6 and 1.7).
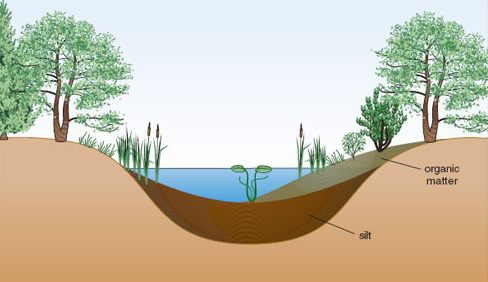

Nutrient enrichment occurs through addition of sediment, rainfall and the decay of resident animals and plants and their excreta. Starting from an oligotrophic state with low productivity, a typical temperate lake increases in productivity fairly quickly as nutrients accumulate, before reaching a steady state of eutrophy which might last for a very long time (perhaps thousands of years). However, it is possible for the nutrient status of a water body to fluctuate over time and for trophic state to alter accordingly. Study of sediments in an ancient lake in Japan, Lake Biwa (believed to be around four million years old) suggests that it has passed through two oligotrophic phases in the last half million years, interspersed with two mesotrophic phases and one eutrophic phase. Evidence such as this has led to the suggestion that the nutrient status of lakes reflects contemporary nutrient supply, and can increase or decrease in response to this. The processes by which nutrients are washed downstream or locked away in sediments help to ensure that reversal of natural eutrophication can occur.
Rivers vary in trophic state between source and sea, and generally become increasingly eutrophic as they approach sea-level.
1.4 Human-induced eutrophication
While eutrophication does occur independently of human activity, increasingly it is caused, or amplified, by human inputs. Human activities are causing pollution of water bodies and soils to occur to an unprecedented degree, resulting in an array of symptomatic changes in water quality and in species and communities of associated organisms. In 1848 W. Gardiner produced a flora of Forfarshire, in which he described the plants growing in Balgavies Loch. He talked of ‘potamogetons [pondweeds] flourishing at a great depth amid the transparent waters, animated by numerous members of the insect and finny races’. These ‘present a delightful spectacle, and the long stems of the white and yellow water lilies may be traced from their floating flowers to the root’. By 1980, the same loch had very low transparency and dense growths of planktonic algae throughout the summer. The submerged plants grew no deeper than 2 m, and in the 1970s included just three species of Potamogeton, where previously there were 17.
For any ecosystem, whether aquatic or terrestrial, nutrient status plays a major part in determining the range of organisms likely to occur. Characteristic assemblages of plant and associated animal species are found in water with different trophic states. Table 1.3 shows some of the aquatic macrophyte species associated with different concentrations of phosphorus in Britain.
| Phosphorus present as soluble reactive phosphorus (SRP) Footnotes */mg P l-1 | Plant species (see Figure 1.8 for illustrations) |
|---|---|
| bog pondweed, Potamogeton polygonifolius river water-crowfoot,Ranunculus fluitans | |
| 0.1-0.4 | fennel-leaved pondweed, Potamogeton pectinatus |
| 0.4-1.0 | yellow water-lily,Nuphar lutea arrowhead,Sagittarias agittifolia |
| >1.0 | spiked water-milfoil, Myriophyllum spicatum |
Footnotes
* This term is explained in Section 2.1. Back to main textSAQ 6
What impression would you gain from an observation that a population of river water-crowfoot in a particular stretch of river had been largely replaced by fennel-leaved pondweed over a three-year period?
Answer
The phosphorus concentration of the water may have increased.

Figure 1.9 illustrates the relationship between levels of total phosphorus in standing water and the nutrient status of lakes. Above a level of 0.1 mg phosphorus per litre, biodiversity often declines. Using the trophic bands defined in Table 1.2, this is the concentration at which lakes are considered to become hypertrophic. This is way below the standard of 50 mg l−1 set as the acceptable limit for phosphorus in drinking water. Nutrient loadings this high are generally caused by human activities. Extremely high levels of eutrophication are often associated with other forms of pollution, such as the release of toxic heavy metals, resulting in ecosystems that may no longer support life (Figure 1.10).
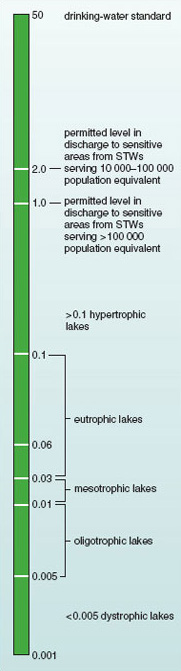

Figure 1.10
For lakes with no written historical records, the diatom record of sediments can be used to study earlier periods of natural change in water quality, and to provide a baseline against which to evaluate trends in artificial or human-induced eutrophication. Diatoms are microscopic photosynthetic organisms (algae of the kingdom Protoctista), which live either free-floating in lakes or attached to the surface of rocks and aquatic vegetation. It is well established that some species of diatom can tolerate oligotrophic conditions whereas others flourish only in more eutrophic waters. When they die, their tiny (
Studies of diatom remains have demonstrated that current levels of eutrophication far exceed those found historically. In the English Lake District, productivity and sediment input increased in some lakes when vegetation was cleared by Neolithic humans around 5000 years ago, and again when widespread deforestation occurred 2000 years ago. However the greatest increases in productivity, sediment levels and levels of carbon, nitrogen and phosphorus, have occurred since 1930. Figure 1.11 shows the general pattern of changes in productivity in Cumbrian lakes through history as the type and intensity of human activities has changed.
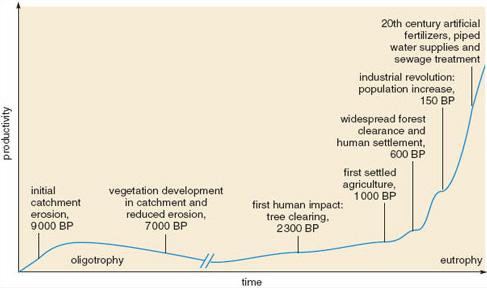
Figure 1.11
In the Norfolk Broads, the waters of the River Ant had a diverse macrophyte flora during the 19th century. The submerged species known as water soldier (Stratiotes aloides, Figure 1.12) was common, but by 1968 the only macrophytes remaining were those with permanently floating leaves, such as water-lilies. During that period, throughout the Broads, there was a general trend away from clear-water habitats, typified by, for example, the diminutive angiosperm known as the holly-leaved naiad (Najas marina), towards habitats containing more productive species, such as pondweeds (Potamogeton spp.) andhornworts (Ceratophyllum spp.). In some cases, they eventually became eutrophic habitats with turbid water, typified by free-floating green algae and cyanobacteria, with very few macrophytes at all. For example, hornwort (Ceratophyllum demersum) was almost choking Alderfen Broad in 1963, but had almost disappeared by 1968 to be replaced eventually by algal blooms in the 1990s.

Figure 1.12
Sediment cores from the River Ant and neighbouring broads suggest that observed changes in plant community composition were linked to rising levels of total phosphorus: mean levels in the area rose dramatically between 1900 and 1975 (Figure 1.13), but have since fallen as a result of actions taken to remove phosphorus from the system.
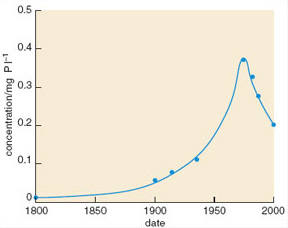
Figure 1.13
SAQ 7
Using the trophic bands in Table 1.2, describe the change in the River Ant broads between 1800 and 1975.
Answer
In 1800 the water was at the upper end of the oligotrophic range; it had moved through the mesotrophic range to become eutrophic by 1900, and by 1940 would be classed as hypertrophic. Between 1940 and 1975 there was a further threefold increase in the concentration of total phosphorus.
Eutrophication has damaged a large number of sites of special scientific interest (SSSIs) designated in the UK under the Wildlife and Countryside Act of 1981: English Nature has identified a total of 90 lake SSSIs and 12 river SSSIs that have been adversely affected. Artificial eutrophication in rivers is even more widespread than in lakes and reservoirs. Human activities worldwide have caused the nitrogen and phosphorus content of many rivers to double and, in some countries, local increases of up to 50 times have been recorded.
Eutrophication has also become a problem for terrestrial wildlife. Deposition of atmospheric nitrogen and the use of nitrogen-rich and phosphorus-rich fertilizers in agriculture has resulted in nutrient enrichment of soils and has caused associated alteration of terrestrial plant and animal communities.
Some of the effects of large-scale eutrophication have adverse consequences for people, and efforts to manage or reduce eutrophication in different countries now cost substantial sums of money. Removing nitrates from water supplies in England and Wales cost £20 million in 1995. Higher frequency of algal blooms increases the costs of filtration for domestic water supply and may cause detectable tastes and odours due to the secretion of organic compounds. If the bloom is large, these compounds can accumulate to concentrations that are toxic to mammals and sometimes fish. Furthermore, the high productivity of the blooms means that although oxygen is released by photosynthesis during the day, the effect of billions of cells respiring overnight can deplete the water of oxygen, resulting in fish dying through suffocation even if they tolerate the toxins.
SAQ 8
Are fish most at risk from suffocation in warm or cool water?
Answer
Warm water, because oxygen is less soluble at warmer temperatures and is therefore more rapidly depleted by respiring organisms, especially as respiration rate also increases with temperature.
Another problem caused to the water industry by algal blooms is the production of large quantities of fine organic detritus, which, when collected within waterworks’ filters, may support clogging communities of aquatic organisms such as nematode worms, sponges and various insects. These may subsequently find their way into water distribution pipes and on occasion appear in tap water!
2 Effects of eutrophication
Introduction
A number of biological changes may occur as a result of eutrophication. Some of these are direct (e.g. stimulation of algal growth in water bodies), while others are indirect (e.g. changes in fish community composition due to reduced oxygen concentrations). This section summarizes some of the typical changes observed in aquatic, marine and terrestrial ecosystems following eutrophication.
Some typical changes observed in lakes following artificial eutrophication are summarized in Table 2.1. Similar characteristic changes are observed in other freshwater systems.
| • Turbidity increases, reducing the amount of light reaching submerged plants. |
| • Rate of sedimentation increases, shortening the lifespan of open water bodies such as lakes. |
| • Primary productivity usually becomes much higher than in unpolluted water and may be manifest as extensive algal or bacterial blooms. |
| • Dissolved oxygen in water decreases, as organisms decomposing the increased biomass consume oxygen. |
| • Diversity of primary producers tends to decrease and the dominant species change. Initially the number of species of green algae increases, causing temporary increase in diversity of primary producers. However, as eutrophication proceeds, blue-green bacteria become dominant, displacing many algal species. Similarly some macrophytes (e.g. bulrushes) respond well initially, but due to increased turbidity and anoxia (reduced oxygen) they decline in diversity as eutrophication proceeds. |
| • Fish populations are adversely affected by reduced oxygen availability, and the fish community becomes dominated by surface-dwelling coarse fish, such as pike (Esox lucius, see Figure 2.1) and perch (Perca fluviatilis). |
| • Zooplankton (e.g. Daphnia spp.), which eat phytoplankton, are disadvantaged due to the loss of submerged macrophytes, which provide their cover, thereby exposing them to predation. |
| • Increased abundance of competitive macrophytes (e.g. bulrushes) may impede water flow, increasing rates of silt deposition. |
| • Drinking water quality may decline. Water may be difficult to treat for human consumption, for example due to blockage of filtering systems. Water may have unacceptable taste or odour due to the secretion of organic compounds by microbes. |
| • Water may cause human health problems, due to toxins secreted by the abundant microbes, causing symptoms that range from skin irritations to pneumonia. |
In oligotrophic systems, even quite small increases in nutrient load can have relatively large impacts on plant and animal communities.

2.1 Effects on primary producers in freshwater ecosystems
Plant species differ in their ability to compete as nutrient availability increases. Some floating and submerged macrophyte species are restricted to nutrient-poor waters, while others are typical of nutrient-rich sites (see Table 2.2). Figure 2.2 shows turbid water in a polluted drainage ditch associated with localized growth of algae. There are no aquatic plants present.
| Trophic state | Associated macrophytespecies |
|---|---|
| oligotrophic | alternate water-milfoil (Myriophyllum alternifolium) bog pondweed (Potamogeton polygonifolius) |
| oligo-mesotrophic | bladderwort (Utricularia vulgaris) |
| eutrophic | hairlike pondweed (Potamogeton trichoides) |
| tending towards hypertrophic | spiked water-milfoil (Myriophyllum spicatum) fennel-leaved pondweed (Potamogeton pectinatus) |

In rivers, the presence of plant species such as the yellow water-lily (Nuphar lutea) and the arrowhead (Sagittaria sagittifolia, Figure 2.3) are likely to indicate eutrophic conditions. In some rivers, the fennel-leaved pondweed (Potamogeton pectinatus) is tolerant of both sewage and industrial pollution.

Whereas some species can occur in waters with quite a wide range of nutrient levels, some are relatively obligate to specific trophic bands and are unable to survive if nutrient levels alter significantly from those to which they are adapted. In 1989, Michael Jeffries derived ranges of tolerance for a number of macrophyte species by studying literature on their occurrence and distribution in relation to different aspects of water quality. He also reviewed results of scientific studies reported in the literature to determine what concentrations of nitrate, ammonia, phosphorus, suspended solids and biological oxygen demand (BOD) appeared to be associated with severe or total loss of macrophyte species due to eutrophication (see Table 2.3). Research has suggested that changes to certain macrophyte communities can occur at soluble reactive phosphorus concentrations as low as 20 μg 1(1(0.02 mg l(1). Soluble reactive phosphorus (SRP) is the term commonly used to describe phosphorus that is readily available for uptake by organisms. It is used in contrast to measures of total phosphorus, which include forms of the element that are bound to sediment particles or locked up in large organic molecules. These forms are unavailable for immediate uptake, but they may become available over time.
Question 2.1
Water samples from two lowland rivers, A and B, are found to contain the following concentrations of plant nutrients.
| Nutrient | Concentration/mg l-1 | |
|---|---|---|
| River A | River B | |
| nitrate | 2.2 | 12.1 |
| ammonia | 0.07 | 0.6 |
| SRP | 0.18 | 0.13 |
By reference to Table 2.3, what conclusions can you draw about the probable diversity of aquatic macrophytes in each of the rivers?
| Condition | SRP/mg P l−1 | Nitrate/mg N l−1 | Ammonia/mg N l−1 | Suspended solids | BOD |
|---|---|---|---|---|---|
| ‘natural’ | |||||
| degraded (partial loss of species found under ‘natural’ conditions) | 0.1-0.2 | 3.0-10 | 0.2-5.0 | 30-100 | 2.0-6.0 |
| severe loss of species | >0.2 | >10 | >5.0 | >100 | >6.0 |
Footnotes
BOD, biological oxygen demand; SRP, soluble reactive phosphorus.
Answer
River A has a soluble reactive phosphorus concentration in the range 0.1-0.2 mg 1−1, which corresponds to the ‘degraded’ category in Table 2.3. This suggests that the diversity of macrophytes would be less than in the pristine natural state, due to a limited eutrophication effect. Neither form of nitrogen is present at concentrations above the natural range, so primary productivity may become limited by nitrogen rather than phosphorus, limiting the impact of the elevated phosphorus concentration.
River B has a similar concentration of SRP to river A, which would again place it in the ‘degraded’ category, but a much higher concentration of nitrogen in both its forms, especially nitrate at 12.1 mg 1−1, taking it into the ‘severe loss of species’ category. The elevated availability of both P and N would boost primary production in the watercourse, favouring algal communities and leading to a decline in macrophyte populations and diversity. The more competitive macrophytes may benefit from the increased nutrient availability, but their increased growth would further exclude less competitive species, resulting in lower diversity.
2.1.1 Loss of submerged plant communities
One of the symptoms of extreme eutrophication in shallow waters is often a substantial or complete loss of submerged plant communities and their replacement by dense phytoplankton communities (algal blooms). This results not only in the loss of characteristic plant species (macrophytes) but also in reduced habitat structure within the water body. Submerged plants provide refuges for invertebrate species against predation by fish. Some of these invertebrate species are phytoplankton-grazers and play an important part in balancing relative proportions of macrophytes and phytoplankton. Submerged macrophytes also stabilize sediments and the banks of slow-flowing rivers or lakes. Bodies of water used for recreation (boating for example) become more vulnerable to bank destabilization and erosion in the absence of well-developed plant communities, making artificial bank stabilization necessary (Figure 2.4). Submerged plants also have a role in the oxygenation of lower water layers and in the maintenance of aquatic pH.

SAQ 9
Name three species of submerged macrophytes that are tolerant of eutrophic water.
Answer
Spiked water-milfoil (Myriophyllum spicatum), fennel-leaved pondweed (Potamogeton pectinatus) and arrowhead (Sagittaria sagittifolia).
2.1.2 Algal blooms
The enrichment of water bodies by eutrophication may be followed by population explosions or ‘blooms’ of planktonic organisms.
SAQ 10
Bursts of primary production in an aquatic ecosystem in response to an increased nutrient supply are commonly referred to as ‘algal blooms’. Can you explain why this term is taxonomically incorrect?
Answer
The organisms responsible may be either algae or bacteria, or a mixture of the two. It is incorrect to refer to bacteria as algae as they belong to a completely different taxonomic kingdom.
‘Algal blooms’ are a well-publicized problem associated with increased nutrient levels in surface waters. The higher the concentration of nutrients, the greater the primary production that can be supported. Opportunistic species like some algae are able to respond quickly, showing rapid increases in biomass. Decomposition of these algae by aerobic bacteria depletes oxygen levels, often very quickly. This can deprive fish and other aquatic organisms of their oxygen supply and cause high levels of mortality, resulting in systems with low diversity. The odours associated with algal decay taint the water and may make drinking water unpalatable. Species of cyanobacteria that flourish in nutrient-rich waters can produce powerful toxins that are a health hazard to animals. Such problems are well documented for a number of famous lakes. The Zurichsee in Switzerland has been subject to seasonal blooms of the cyanobacterium Oscillatoria rubescens due to increased sewage discharge from new building developments on its shores. For lakes in Wisconsin, USA, ‘nuisance’ blooms of algae or bacteria occur whenever concentrations of phosphate and nitrate rise.
2.2 Effects on consumers in freshwater ecosystems
Increased productivity tends to increase rates of deoxygenation in the surface layer of lakes. Although phytoplankton release oxygen to the water as a byproduct of photosynthesis during the day, water has a limited ability to store oxygen and much of it bubbles off as oxygen gas. At night, the phytoplankton themselves, the zooplankton and the decomposer organisms living on dead organic matter are all respiring and consuming oxygen. The store of dissolved oxygen thus becomes depleted and diffusion of atmospheric oxygen into the water is very slow if the water is not moving.
SAQ 11
What s the relative rate of oxygen diffusion in water compared with its rate in air?
Answer
Oxygen diffuses through water at approximately one ten-thousandth of its rate through air.
Still waters with high productivity are therefore likely to become anoxic.
Figures 2.5 and 2.6 give an example of the change in aquatic invertebrate species following eutrophication. In unpolluted water, mayfly larvae may be found. In polluted water, these species cannot survive due to reduced oxygen availability and are likely to be replaced by species, such as the bloodworm, which can tolerate lower oxygen concentrations.

Many species of coarse fish, such as roach (Rutilus rutilus, a cyprinid fish, Figure 2.7), can also tolerate low oxygen concentrations in the water, sometimes gulping air, and yields of fish may indeed increase due to the high net primary production (NPP) of the system. However these species are generally less desirable for commercial fishing than others such as salmon (Salmo salar, a salmonid fish, Figure 2.8), which depend on cool, well-oxygenated surface water. Populations of such species usually decline in waters that become eutrophic (Figure 2.9); they may be unable to live in a deoxygenated lake at all, resulting in fish kills (Figure 2.10). They may also be unable to migrate through deoxygenated waters to reach spawning grounds, resulting in longer-term population depressions.




Figure 2.10
Lake Victoria is one of the world’s largest lakes and used to support diverse communities of species endemic to the lake (i.e. species that are found only there), but it now suffers from frequent fish kills caused by episodes of deoxygenation. In the 1960s deoxygenation was limited to certain areas of the lake, but it is now widespread. It is usually associated with at least a tenfold increase in the algal biomass and a fivefold increase in primary productivity.
When eutrophication reaches a stage where dense algal growth outcompetes marginal aquatic plants, even relatively tolerant fish species suffer from the consequent loss of vegetation structure, especially young fish (Figure 2.11). Spawning is reduced for fish species that attach their eggs to aquatic plants or their detritus, and fish that feed on large plant-eating invertebrates, such as snails and insect nymphs, suffer a reduced food supply.

Figure 2.11
SAQ 12
In a southeast Asian village where cyprinid fish from the local pond are an important source of protein, eutrophication of the water by domestic sewage is seen as advantageous. Why?
Answer
The cyprinid fish are tolerant of deoxygenation, and the increased NPP boosts their food supply; therefore the yield of fish improves.
2.3 Effects on terrestrial vegetation
SAQ 13
Why do you think nitrogen is becoming increasingly available to terrestrial ecosystems in many parts of the world)
Answer
Emission of nitrogen oxides from burning fossil fuel and of ammonia from intensive agriculture result in nitrogen compounds being transported and deposited by atmospheric processes.
Some suggested global scenarios for the year 2100 identify nitrogen deposition (together with land use and climate change) as one of the most significant ‘drivers’ of biodiversity change in terrestrial ecosystems.
Atmospheric deposition of nitrogen, together with the deposition of phosphorus-rich sediments by floods, can alter competitive relationships between plant species within a terrestrial community. This can cause significant changes in community composition, as species differ in their relative responses to elevated nutrient levels. As is the case with aquatic vegetation, terrestrial species that are able to respond to extra nitrogen and phosphorus with elevated rates of photosynthesis will achieve higher rates of biomass production, and are likely to become increasingly dominant in the vegetation. Atmospheric deposition of nutrients can reduce, or even eliminate, populations of species that have become adapted to low nutrient conditions and are unable to respond to increased nutrient availability. Some vegetation communities of conservation interest are directly threatened by atmospheric pollution.
In Britain, rare bryophytes are found associated with snowbeds (Figure 2.12). Most of the late-lying snowbeds in Britain are in the Central Highlands of Scotland, which are also areas of very high deposition of nitrogenous air pollutants. Snow is a very efficient scavenger of atmospheric pollution and melting snowbeds release their pollution load at high concentrations in episodes known as ‘acid flushes’. The flush of nitrogen is received by the underlying vegetation when it has been exposed following snowmelt. Concentrations of nutrients in the meltwater of Scottish snowbeds have already been shown to damage underlying bryophytes, including a rare species called Kiaeria starkei. Recovery from damage is slow, and sometimes plants show no signs of recovery even four weeks after exposure to polluted meltwater. Given the very short growing season, this persistent damage can greatly reduce the viability and survival of the plants. Tissue nitrogen concentrations in Kiaeria starkei have been shown to be up to 50% greater than that recorded in other upland bryophytes. This example emphasizes the potential threat of atmospheric pollution to snowbed species, and suggests that some mountain plant communities may receive much higher pollution loadings than was previously realized.

Figure 2.12
The deposition of atmospheric nitrogen can be enhanced at high altitude sites as a consequence of cloud droplet deposition on hills. Sampling of upland plant species at sites in northern Britain has shown marked increases in nitrogen concentration in leaves with increasing nitrogen deposition, which is, in turn, correlated with increasing altitude. The productivity of the species was also found to increase in line with the amount of nitrogen deposited. Plant species can therefore respond directly to elevated levels of nitrogen. In the longer term, the relative dominance of species is likely to alter depending on their ability to convert elevated levels of deposited nitrogen into biomass.
SAQ 14
What will be the effect on species diversity of increasing biomass?
Answer
As biomass increases beyond an optimal value, species diversity will decline.
Atmospheric pollution can also affect plant-insect interactions. Unusual episodes of damage to heather moorland in Scotland have been caused by the winter moth (Operophtera brumata) in recent years. It has been suggested that this may be due to the effects of increased nitrogen supply on heather plants, including increased shoot growth and a decrease in the carbon : nitrogen ratio in plant tissues. Winter moth larvae have been shown to grow faster on nitrogen-treated heather plants, so it is possible that increased atmospheric deposition of nitrogen may have a role in winter moth outbreaks and the associated degradation of heather moorland in upland Britain.
Although uplands are more susceptible to atmospheric deposition of nitrogen, the effects can be seen in lowland areas too. Nitrogen deposition and the consequent eutrophication of ecosystems is now regarded as one of the most important causes of decline in plant species in the Netherlands. Figure 2.13 shows how the number of grassland species of conservation interest in south Holland declines as the nitrogen load increases. The maximum percentage of species (approximately 95%) is possible at a nitrogen load of about 6 kg N ha−1 yr−1. At loads higher than 10 kg N ha−1 yr−1 the number of species declines due to eutrophication effects, and below 5 kg ha−1 yr−1nitrogen may be too limiting for a few species.
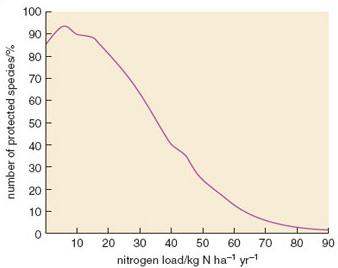
Figure 2.13
A significant proportion of important nature conservation sites in Britain are subject to nitrogen and/or sulfur deposition rates that may disturb their biological communities. Lowland heath ecosystems, for example, have a high profile for conservation action in Britain. They typically have low soil nutrient levels and a vegetation characterized by heather (Calluna vulgaris). Under elevated atmospheric deposition of nitrogen, they tend to be invaded by taller species, including birch (Betula spp., Figure 2.14), bracken (Pteridium aquilinum) and the exotic invader, rhododendron (Rhododendron ponticum).

Figure 2.14
A large number of SSSIs in the UK, designated as such on account of their terrestrial plant communities, are considered to have been damaged by eutrophication. This has been identified as a factor in the decline of some important UK habitats, including some identified for priority action under the UK’s Biodiversity Action Plan (BAP). Wet woodlands, for example, occur on poorly drained soils, usually with alder, birch and willow as the predominant tree species, but sometimes oak, ash or pine occur in slightly drier locations. These woodlands are found on floodplains, usually as a successional habitat on fens, mires and bogs, along streams and in peaty hollows. They provide an important habitat for a variety of species, including the otter (Lutra lutra, Figure 2.15), some very rare beetles and craneflies. They also provide damp microclimates, which are particularly suitable for bryophytes, and have some unusual habitat features not commonly found elsewhere, such as log jams in streams which support a rare fly, Lipsothrix nigristigma. Wet woodlands occur on a range of soil types, including relatively nutrient-rich mineral soils as well as acid, nutrient-poor ones. Nevertheless, many have been adversely affected by eutrophication, resulting in altered ground flora composition and changes in the composition of invertebrate communities.

Nutrient enrichment can also affect habitats found in drier sites. Eutrophication caused by runoff from adjacent agricultural land has been identified as a cause of altered ground flora composition in upland mixed ash woods for example. These woods are notable for bright displays of flowers such as bluebell (Hyacinthoides non-scripta), primrose (Primula vulgaris) and wild garlic (Allium ursinum, Figure 2.16a). They also support some very rare woodland flowers which are largely restricted to upland ash woods, such as dark red helleborine (Epipactis atrorubens, Figure 2.16b) and Jacob’s ladder (Polemonium caeruleum).

Figure 2.16
Other terrestrial UK BAP habitats that may be adversely affected by nutrient enrichment from agricultural fertilizers or atmospheric deposition include lowland wood pasture, lowland calcareous grassland, upland hay meadows and lowland meadows; again the result can be altered plant species composition.
Coastal marshes and wetlands in many parts of the world have been affected by invasion of ‘weed’ or ‘alien’ species. Eutrophication can accelerate invasion of aggressive, competitive species at the expense of slower growing native species. In the USA, many coastal marshes have been invaded by the common reed (Phragmites australis, Figure 2.17). Phragmites is a fierce competitor and can outcompete and entirely displace native marsh plant communities, causing local extinction of plants and the insects and birds that feed on them. Phragmites can spread by underground rhizomes and can rapidly colonize large areas. However, it is the target of conservation effort in some areas, including Britain, because the reedbeds it produces provide an ideal habitat for rare bird species such as the bittern (Botaurus stellarus). But its spread is not always beneficial for nature conservation, as it often results in the drying of marsh soils, making them less suitable for typical wetland species and more suitable for terrestrial species. This is because Phragmites is very productive and can cause ground levels to rise due to deposition of litter and the entrapment of sediment. Thus eutrophication can also play an indirect part in the loss of wetland habitats.

Figure 2.17
2.4 Effects on marine systems
In the marine environment, nutrient enrichment is suspected when surface phytoplankton blooms are seen to occur more frequently and for longer periods. Some species of phytoplankton release toxic compounds and can cause mass mortality of other marine life in the vicinity of the bloom. Changes in the relative abundance of phytoplankton species may also occur, with knock-on effects throughout the food web, as many zooplankton grazers have distinct feeding preferences. In sheltered estuarine areas, high nutrient levels appear to favour the growth of green macroalgae (‘seaweeds’) belonging to such genera as Enteromorpha and Ulva (Figure 2.18).
2.4.1 Estuarine species
Nutrient runoff from the land is a major source of nutrients in estuarine habitats. Shallow-water estuaries are some of the most nutrient-rich ecosystems on Earth, due to coastal development and the effects of urbanization on nutrient runoff. Figure 2.19 shows some typical nitrogen pathways. Nitrogen loadings in rainfall are typically assimilated by plants or denitrified, but septic tanks tend to add nitrogen below the reach of plant roots, and if situated near the coast or rivers can lead to high concentrations entering coastal water. Freshwater plumes from estuaries can extend hundreds of kilometres offshore (Figure 2.20) and the nutrients within them have a marked effect on patterns of primary productivity. Localized effects of eutrophication can be dramatic. For example, increased nitrogen supplies lead to the replacement of seagrass beds (e.g. Zostera marina) by free-floating rafts of ephemeral seaweeds such as Ulva and Cladophera, whose detritus may cover the bottom in a dense layer up to 50 cm thick.
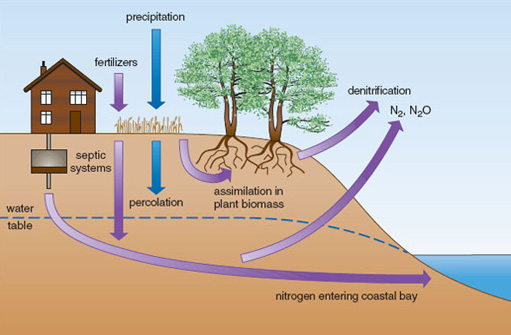
Figure 2.19
Figure 2.20
Estuarine waters enriched by nitrogen from fertilizers and sewage have been responsible for the decline of a number of estuarine invertebrate species, often by causing oxygen depletion of bottom water. Intertidal oyster beds have declined considerably as a result of both over-harvesting and reduced water quality. Harvesting tends to remove oysters selectively from shallow-water habitats, reducing the height of oyster beds and making remaining oysters more vulnerable to the damaging effects of eutrophication. In estuaries, elevated rates of microbial respiration deplete oxygen, and periods of anoxia occur more frequently, especially in summer when water temperatures are high and there is slow water circulation. Oysters in deeper water are more likely to be exposed to anoxic conditions, being further removed from atmospheric oxygen inputs, and to die as a result.
Seagrass distributions are very sensitive to variation in light. Seagrasses, like any other plant, cannot survive in the long term if their rate of photosynthesis is so limited by light that it cannot match their rate of respiration. Light transmission is a function of water column turbidity (cloudiness), which in turn is a combination of the abundance of planktonic organisms and the concentration of suspended sediment. Seagrass distributions are therefore strongly affected by eutrophication and effects on water clarity. In Chesapeake Bay, eastern USA, seagrass beds historically occurred at depths of over 10 m. Today they are restricted to a depth of less than 1 m. Runoff from organic fertilizers has increased plankton production in the water column, limiting light transmission. In addition, large areas of oyster bed have been lost (also partly as a result of eutrophication) with an associated reduction in natural filtration of bay water. Oysters, like many shellfish, clean the water while filtering out microbes, which they then consume. Seagrass beds now cover less than 10% of the area they covered a century ago (Figure 2.21). Similar patterns have occurred elsewhere.
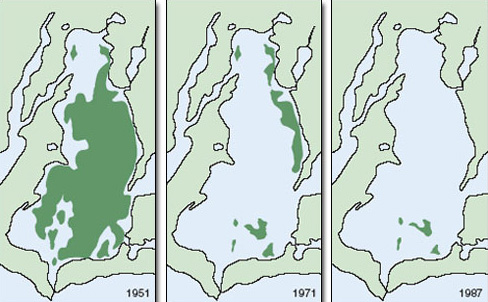
Figure 2.21
Seagrass beds play an important role in reducing the turbidity of coastal waters by reducing the quantity of sediment suspended in the water. Seagrasses slow down currents near the bottom, which increases the deposition of small sediment particles and decreases their erosion and resuspension. Seagrass roots also play an important part in stabilizing sediments and limiting disturbance caused by burrowing deposit-feeders.
Many species depend on seagrass beds for food or nursery grounds. Seagrass increases the structural complexity of habitat near the sea floor, and provides a greater surface area for epiphytic organisms. Seagrass leaves support rich communities of organisms on their surface, including microalgae, stationary invertebrates (such as sponges and barnacles) and grazers (such as limpets and whelks). The plants also provide refuges from predatory fishes and crabs. Predation on seagrass-associated prey such as the grass shrimp is much higher outside seagrass beds than within them, where the shrimps can hide and predator foraging is inhibited by grass cover. Without seagrasses, soft-substrate communities on the sea bed are simpler, less heterogeneous and less diverse. By reducing the health of seagrasses, eutrophication contributes directly to biotic impoverishment (Figure 2.22).
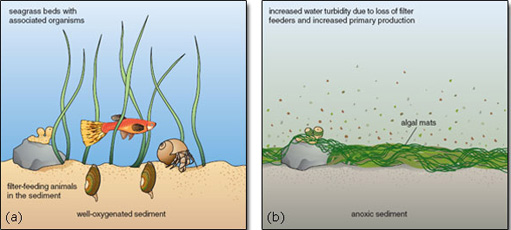
Figure 2.22
Question 2.2
Seagrass (Zostera marina) is a key species for maintaining the biodiversity of estuaries. Why is its abundance reduced following eutrophication of estuarine waters? Can you identify a mechanism in which the decline of seagrass promotes its further decline?
Answer
Seagrass needs sufficient light to photosynthesize effectively. An increase in nutrient levels leads to a greater abundance of phytoplankton in the water column, which increases the turbidity of the water and blocks out the light. As the seagrass beds recede, the exposed sediment may be re-suspended and further increase the turbidity of the water, thereby exacerbating the problem in a classic positive feedback response. Another possible positive feedback loop is the loss of habitat for filter-feeding animals, which lived in the shelter of seagrass beds and helped keep the water clear by consuming microbes.
2.4.2 Saltmarshes
Marsh plant primary production is generally nitrogen limited, so saltmarsh vegetation responds readily to the artificial eutrophication that is now so common in nearshore waters. Eutrophication causes marked changes in plant communities in saltmarshes, just as it does in freshwater aquatic and terrestrial systems. Biomass production increases markedly as levels of eutrophication increase. Increases in the nitrogen content of plants cause dramatic changes in populations of marsh plant consumers: insect herbivores tend to increase (Figure 2.23) and so do numbers of carnivorous insects. Thus, increasing the nitrogen supply to saltmarshes has a dramatic bottom-up effect on marsh food webs. Eutrophication can also alter the outcome of competition among marsh plants, by changing the factor limiting growth. At low levels of nitrogen, plants that exploit below-ground resources most effectively, such as the saltmarsh rush (Juncus gerardii) are competitively dominant, but at higher nutrient levels dominance switches to plants that are good above-ground competitors, such as the common cord grass (Spartina anglica, Figure 2.24). In other words, as nitrogen availability increases, competition for light becomes relatively more important.
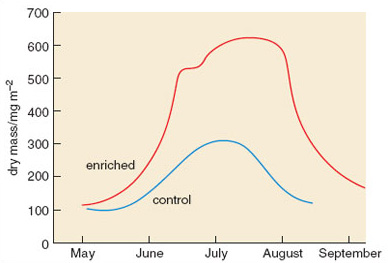
Figure 2.23

Figure 2.24
3 Causes and mechanisms of eutrophication
3.1 Agents of eutrophication
Light availability, water availability, temperature and the supply of plant nutrients are the four most important factors determining NPP. Altered availability of nutrients affects the rate of primary production in all ecosystems, which in turn changes the biomass and the species composition of communities.
SAQ 15a
Which two elements most often limit NPP?
Answer
Phosphorus and nitrogen are the main limiting nutrients.
Compounds containing these elements are therefore the causal agents of eutrophication in both aquatic and terrestrial systems. Let us consider them in turn.
3.1.1 Phosphorus
Phosphorus has a number of indispensable biochemical roles and is an essential element for growth in all organisms, being a component of nucleic acids such as DNA, which hold the code for life. However, phosphorus is a scarce element in the Earth’s crust and natural mobilization of phosphorus from rocks is slow. Its compounds are relatively insoluble, there is no reservoir of gaseous phosphorus compounds available in the atmosphere (as there is for carbon and nitrogen), and phosphorus is also readily and rapidly transformed into insoluble forms that are unavailable to plants. This tends to make phosphorus generally unavailable for plant growth. In natural systems, phosphorus is more likely to be the growth-limiting nutrient than is nitrogen, which has a relatively rapid global cycle and whose compounds tend to be highly soluble.
Human activities, notably the mining of phosphate-rich rocks and their chemical transformation into fertilizer, have increased rates of mobilization of phosphorus enormously. A total of 12 × 1012 g P yr−1 are mined from rock deposits. This is six times the estimated rate at which phosphorus is locked up in the ocean sediments from which the rocks are formed. The global phosphorus cycle is therefore being unbalanced by human activities, with soils and water bodies becoming increasingly phosphorus-rich. Eutrophication produces changes in the concentrations of phosphorus in all compartments of the phosphorus cycle.
The mechanisms of eutrophication caused by phosphorus vary for terrestrial and aquatic systems. In soils, some phosphorus comes out of solution to form insoluble iron and aluminium compounds, which are then immobilized until the soil itself is moved by erosion. Eroded soil entering watercourses may release its phosphorus, especially under anoxic conditions.
SAQ 16
What changes occur to iron(III) compounds (Fe3+) as a result of bacterial respiration in anoxic environments, and how is their solubility affected?
Answer
Bacterial respiration can reduce Fe3+ to Fe2+, increasing the solubility of iron salts, including phosphates of iron.
Once in rivers, retention times for phosphorus may be short, as it is carried downstream either in soluble form or as suspended sediment. Algal blooms are therefore less likely to occur in moving waters than in still systems. In the latter, there is more time for the phosphorus in enriched sediments to be released in an ‘available’ form, increasing the concentration of soluble reactive phosphorus (SRP), and thus affecting primary production.
Phosphorus is generally acknowledged to be the nutrient most likely to limit phytoplankton biomass, and therefore also the one most likely to cause phytoplankton blooms if levels increase. However, there do appear to be some systems that are ‘naturally eutrophic’, with high phosphorus loadings. In these systems, nitrogen concentrations may then become limiting and play a dominant role in determining phytoplankton biomass.
3.1.2 Nitrogen
Nearly 80% of the atmosphere is nitrogen. Despite the huge supply potentially available, nitrogen gas is directly available as a nutrient to only a few organisms.
Why cannot the majority of organisms utilize gaseous nitrogen?
Nitrogen gas is very unreactive and only a limited number of bacterial species have evolved an enzyme capable of cleaving the molecule.
Once ‘fixed’ by these bacteria into an organic form, the nitrogen enters the active part of the nitrogen cycle. As the bacteria or the tissues of their mutualistic hosts die, the nitrogen is released in an available form such as nitrate or ammonium ions - a result of the decay process. Alternatively, the high temperatures generated during electrical storms can ‘fix’ atmospheric nitrogen as nitric oxide (NO). Further oxidation to nitric acid within the atmosphere, and scavenging by rainfall, provides an additional natural source of nitrate to terrestrial ecosystems. Nitrates and ammonium compounds are very soluble and are hence readily transported into waterways.
Nitrogen is only likely to become the main growth-limiting nutrient in aquatic systems where rocks are particularly phosphate-rich or where artificial phosphate enrichment has occurred. However, nitrogen is more likely to be the limiting nutrient in terrestrial ecosystems, where soils can typically retain phosphorus while nitrogen is leached away.
3.2 Anthropogenic sources of nutrients
In addition to the natural sources of nutrients referred to above, nitrogen and phosphorus enter the environment from a number of anthropogenic sources. These are considered below.
3.2.1 The atmosphere
Pollution of the atmosphere has increased rates of nitrogen deposition considerably. Nitrogen has long been recognized as the most commonly limiting nutrient for terrestrial plant production throughout the world, but air pollution has now created a modern, chemical, climate that often results in excess supplies of nitrogen due to atmospheric deposition.
The main anthropogenic source of this enhanced nitrogen deposition is the NOx (mainly as NO) released during the combustion of fossil fuels — principally in vehicles and power plants. Like that generated within the atmosphere, this fixed nitrogen returns to the ground as nitrate dissolved in rainwater.
Patterns and rates of deposition vary regionally, and between urban and rural areas. Concentrations and fluxes of nitrogen oxides tend to decline with distance from cities: deposition of inorganic nitrogen has been found to be twice as high in urban recording sites in New York City than in suburban or rural sites. Some natural ecosystems, particularly those near industrialized areas, now receive atmospheric nitrogen inputs that are an order of magnitude greater than those for pre-industrial times. Figure 3.2 shows intensive industrial land use adjacent to the River Tees and its estuary in Teesside, UK. The estuary is still important for wildlife, including seals and a variety of birds, but its quality has declined markedly due to atmospheric and water pollution. In the UK, atmospheric deposition can add up to 150 kg N ha−1 yr−1. For comparison, the amount thought to trigger changes in the composition of species-rich grassland is 20-30 kg Nha−1 yr−1, and a typical dose farmers apply as inorganic fertiliser to an intensively managed grassland is 100 kg N ha−1 yr−1.


3.2.2 Domestic detergents
Domestic detergents are a major source of phosphorus in sewage effluents. Phosphates are used as a ‘builder’ in washing powders to enhance the efficiency of surfactants by removing calcium and magnesium to make the water ‘softer’. In 1992, the UK used 845 600 tonnes of detergent of various types, all of which have different effects on the environment. Estimates of the relative contribution of domestic detergents to phosphorus build-up in Britain’s watercourses vary from 20-60%. The UK’s Royal Commission on Environmental Pollution (RCEP) reviewed the impacts of phosphate-based detergents on water quality in 1992, focusing on the effects on freshwater. The RCEP concluded that eutrophication was widespread over large parts of the country, and recommended a considerable investment in stripping phosphates from sewage as well as efforts to reduce phosphate use in soft-water areas. The main problem is that many of the ingredients of detergents are not removed by conventional sewage treatment and degrade only slowly.
SAQ 17
Why did the RCEP recommend that phosphate use be reduced particularly in ‘soft’ water areas?
Answer
The reason for including phosphates in detergents is to soften the water, so in areas with naturally soft water they provide no benefit yet still cause pollution.
Other compounds added to detergents may also contribute to eutrophication. Silicates, for example, particularly if used as a partial replacement for phosphates in detergents, can lead to increased growth of diatoms. These algae require silicates to build their ‘skeleton’ and their growth can be limited by silicate availability. When silicates are readily available, diatoms characteristically have ‘spring blooms’ of rapid growth, and can smother the surfaces of submerged macrophytes, depriving them of light. A loss of submerged macrophytes is a problem because it results in the loss of habitat for organisms feeding on phytoplankton, and therefore the risk of blooms by other species is enhanced.
3.2.3 Agricultural fertilizers
Runoff from intensively farmed land often contains high concentrations of inorganic fertilizer. Nutrients applied to farmland may spread to the wider environment by:
drainage water percolating through the soil, leaching soluble plant nutrients;
washing of excreta, applied to the land as fertilizer, into watercourses; and
the erosion of surface soils or the movement of fine soil particles into subsoil drainage systems.
Some water bodies have been monitored for long periods, and the impact of agricultural runoff can be demonstrated clearly. In the 50 years between 1904 and 1954, for example, in Loch Leven, Scotland, there were major changes in the species composition of the community of photosynthetic organisms. The species composition of the green alga community changed and the numbers of cyanobacteria rose considerably. Increasingly since then, large blooms of filamentous cyanobacteria have been produced in the loch. These changes have been linked with trends in the use of agricultural fertilizers and other agrochemicals.
In Europe, large quantities of slurry from intensively reared and housed livestock are spread on the fields (Figure 3.3). Animal excreta are very rich in both nitrogen and phosphorus and therefore their application to land can contribute to problems from polluted runoff. Land use policies have concentrated livestock production into purpose-built units, increasing the pollution risks associated with handling the resultant slurry or manures.

European agricultural policies that subsidize agriculture on the basis of productivity have also encouraged the use of fertilizers. Use of fertilizers has undergone a massive increase since 1950. In the USA, by 1975, total use of inorganic fertilizer had reached a level equivalent to about 40 kg per person per year. A recent European Environment Agency report estimated that the groundwater beneath more than 85% of Europe’s farmland exceeds guideline levels for nitrogen concentration (25 mg l−1), with agricultural fertilizers being the main source of the problem. Pollution of surface waters also occurs on a large scale. A survey by the UK’s Environment Agency in 1994 found that over 50% of the 314 water bodies surveyed in England and Wales had algal blooms caused by fertilizer runoff (Figure 3.4).

Patterns of fertilizer use do differ considerably between countries. In those with poorly developed economies, the costs of artificial fertilizers may be prohibitive. In hotter climates, irrigation may be used, resulting in higher nutrient runoff than for equivalent crops that are not irrigated. The high solubility of nitrate means that agriculture is a major contributor to nitrogen loadings in freshwater. Agriculture accounts for 71% of the mass flow of nitrogen in the River Great Ouse in the Midlands, UK, compared with only 6% for phosphorus.
SAQ 18
What are the main sources of phosphorus and nitrogen that enrich rivers in a developed country
Answer
Phosphorus comes primarily from domestic waste water, whilst nitrogen comes primarily from intensive agriculture.
3.2.4 Land use
Studies evaluating the effects of nutrient loading on receiving water bodies must take account of the range of land uses found within a catchment.
As shown in Table 3.1, phosphate exports increase considerably as forests are converted to agricultural land and as agricultural land is urbanized. Agricultural runoff is known to be a potential source of nutrients for eutrophication, but the degree of mechanization may also be important. In catchments where agriculture is heavily mechanized, higher levels of sedimentation are likely. Most sediments arise as a result of soil erosion, which is promoted by tilling the land intensively. This destroys the soil’s natural structure as well as removing vegetation which helps to stabilize soil.
To cite just one example, high sediment input in the latter half of the 20th century has caused shrinkage of the area of open water in the Mogan Lake system near Ankara, Turkey. Undoubtedly, mechanization and intensification of agriculture have played their part, but so too has the drainage of adjacent wetlands. The drained wetlands no longer trapped sediments, and themselves became vulnerable to erosion. This further increased sediment loadings in the lake. Levels of phosphorus have also risen. Draining the wetlands exposed the organic matter in their soils to oxidation, ‘mobilizing’ the phosphorus that had accumulated there over many years. This was then carried into the lake in drainage water.
| Land use | Total phosphorus | Total nitrogen | |
|---|---|---|---|
| Losses from land to water courses | urban | 0.1 | 0.5 |
| rural/agriculture | 0.05 | 0.5 | |
| forest | 0.01 | 1.3 | |
| Additions to land | atmospheric sources: | ||
| rainfall | 0.02 | 0.8 | |
| dry deposition | 0.08 | 1.6 |
3.2.5 Sediments
Sediments have a variable but complex role in nutrient cycling in most aquatic systems, and are a potential ‘internal’ source of pollutants. Release of phosphorus from lake sediment is a complex function of physical, biological and chemical processes and is not easy to predict for different systems. Nitrogen is not stored and released from sediments in the same way, so its turnover time within aquatic systems is quite rapid. Nitrogen concentrations tend to fall off relatively quickly following a reduction in external nitrogen loading, whilst this is not true for phosphorus because the sediments can hold such a large reservoir of this nutrient that input and output rates may become decoupled.
In some shallow coastal areas, tidal mixing is the dominant nutrient regeneration process, as the sediments are regularly disturbed and redistributed by changing water currents, making nutrient exchange with the water much more rapid.
SAQ 19
Why is a lake in a catchment dominated by arable agriculture much more prone to eutrophication than one in a forested catchment?
Answer
First the arable catchment is likely to be receiving much more nutrient input in the form of fertilizers. Second and equally importantly, the soil structure is much less stable under arable systems and therefore more likely to erode and carry nutrients to the lake as suspended sediment.
3.3 Mechanisms of eutrophication
Direct effects of eutrophication occur when growth of organisms (usually the primary producers) is released from nutrient limitation. The resulting increased NPP becomes available for consumers, either as living biomass for herbivores or as detritus for detritivores. Associated indirect effects occur as eutrophication alters the food supply for other consumers. Changes in the amount, relative abundance, size or nutritional content of the food supply influence competitive relationships between consumers, and hence the relative success and survival of different species. Nutrient-induced changes in plant community composition and productivity can therefore result in associated changes in the competitive balance between herbivores, detritivores and predators. Consumers may also be affected by changes in environmental conditions caused indirectly by eutrophication, for example reduced oxygen concentrations caused by bacterial decay of biomass.
In freshwater aquatic systems, a major effect of eutrophication is the loss of the submerged macrophyte community (Section 2.1.1). Macrophytes are thought to disappear because they lose their energy supply in the form of sunlight penetrating the water. Following eutrophication, the sunlight is intercepted by the increased biomass of phytoplankton exploiting the high availability of nutrients. In principle, the submerged macrophytes could also benefit from increased nutrient availability, but they have no opportunity to do so because they are shaded by the free-floating microscopic organisms. Research in the Norfolk Broads has supported the view that the rapid replacement of diverse macrophyte communities by algal communities is attributable to light attenuation, caused by raised turbidity, but has also suggested that there may be more complex mechanisms operating, which must be understood if practical measures are to be undertaken to tackle eutrophication problems. There is evidence to suggest that either a plant-dominated state or an algal-dominated state can exist under high-nutrient conditions (Figure 3.5). Once either state becomes established, a number of mechanisms come into play which buffer the ecosystem against externally applied change. For example, a well-established submerged plant community may secrete substances that inhibit algal growth, and may provide refuges for animals that graze large quantities of algae. On the other hand, once an algal community becomes well established, especially early in the year, it can shade out the new growth of any aquatic plants on the bottom and compete with them for carbon dioxide in the water.
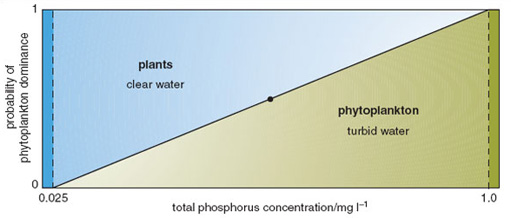
Research in the Norfolk Broads into possible trigger factors for switches from communities dominated by submerged macrophytes to those dominated by algae suggests that pesticides could play a role. Some herbivores are thought to be susceptible to pesticide leaching from surrounding arable land. Pesticide residues in sediments were found at concentrations high enough to cause at least sub-lethal effects, which could reduce the herbivore population for long enough to reduce algal consumption. This could help to explain the observation that most of the Norfolk Broads that have lost their plants are directly connected with main rivers draining intensive arable catchments, whereas those that have retained plant dominance are in catchments where livestock grazing predominates.
Clear relationships can be seen between human population density and total phosphorus and nitrate concentrations in watercourses (Figure 3.6). In 1968 the anthropogenic contribution amounted to some 10.8 g N per capita per day and 2.18 g P per capita per day. Outputs have continued to rise since then. Worldwide, human activities have intensified releases of phosphorus considerably. Increased soil erosion, agricultural runoff, recycling of crop residues and manures, discharges of domestic and industrial wastes and, above all, applications of inorganic fertilizers, are the major causes of this increase. Global food production is now highly dependent on the continuing use of supplementary phosphates, which account for 50-60% of total phosphorus supply.
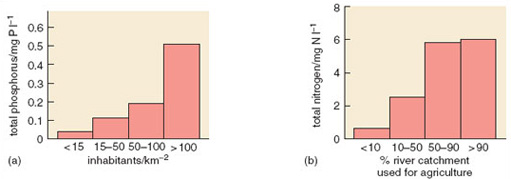
Studies of nutrient runoff have shown a mixture of inputs into most river and lake catchments: both point source (such as sewage treatment works) and diffuse source (such as agriculture). Point sources are usually most important in the supply of phosphorus, whereas nitrogen is more likely to be derived from diffuse sources.
Question 3.1
Using the data presented in Figure 1.13 and Table 2.3, comment on whether the remediation activities on the broads neighbouring the River Ant were likely to have resulted in a recovery of plant species diversity by 2000. Assume that 80% of the total phosphorus in the water is in the form of SRP.
Answer
Figure 1.13 shows that total phosphorus concentrations had fallen to 0.2 mg 1−1 in 2000, compared with their peak concentration of 0.36 mg 1−1 in 1975. In terms of SRP (the form of phosphorus that affects ecosystems most directly), we assume levels have fallen from 0.29 mg 1−1 to 0.16 mg 1−1. Comparing these figures with those in Table 2.3, we see that the SRP concentration put the system in the ‘severe loss of species’ category in 1975, but only the ‘degraded’ category in 2000. This suggests that some recovery of macrophyte species would be possible. Actual re-colonization may be a slow process, however. Ecosystems can take many years to come back to equilibrium after a perturbation, and if an algal-dominated state has established, it will inhibit macrophyte recovery.
4 Managing eutrophication
Introduction
The degree to which eutrophication is considered a problem depends on the place and people concerned. A small lake in South-East Asia, heavily fertilized by village sewage, can provide valuable protein from fish. In other parts of the world, a similar level of nutrients would be regarded as damaging, making water undrinkable and unable to support characteristic wildlife. In Europe, nitrates in drinking water are regarded as a potentially serious threat to health. Eutrophication has also damaged important fisheries and caused significant loss of biodiversity. Worldwide, efforts to reduce the causes and symptoms of eutrophication cost huge sums of money.
There is no single piece of existing legislation dealing comprehensively with the problem of eutrophication in the UK. However, one aim of the European Community’s Urban Wastewater Treatment Directive (EC UWWTD) is to protect the environment from the adverse effects of sewage. This should help to reduce the problem of eutrophication in coastal waters where large discharges contribute significant nutrient loads. In the UK, 62 rivers and canals (totalling 2500 km), 13 lakes and reservoirs and five estuaries have been designated as sensitive areas (eutrophic) under this directive, and there are requirements for reducing nutrient loads from sewage treatment works in these areas (Figure 4.1).
Under the EC UWWTD, areas designated as ‘eutrophic sensitive’ must have phosphorus-stripping equipment installed at sewage treatment works (STWs, Figure 4.2) that serve populations of 10 000 or more. However, the majority of nature conservation sites classified as sensitive are affected by smaller, rural STWs for which such equipment is not yet required. Phosphorus stripping involves the use of chemicals such as aluminium sulfate, which react with dissolved phosphates, causing them to precipitate out of solution.

Another piece of European legislation that has some bearing on problems of nutrient enrichment is the EC Nitrates Directive. This is intended to reduce nitrate loadings to agricultural land, particularly in areas where drinking water supplies have high dissolved nitrate levels. The directive requires member states to monitor nitrate levels in water, set up ‘nitrate vulnerable zones’ (NVZ), and produce and promote a ‘code of good agricultural practice’ throughout the countryside. This should include measures to control the storage, handling and disposal of slurry, for example (Figure 4.3). However, the legislation designed to curb nutrient inputs from agricultural sources is primarily directed towards reducing nitrate levels in drinking water rather than protecting nature conservation sites. The EC Nitrates Directive defines eutrophication only in terms of nitrogen compounds, and therefore does nothing to help protect the majority of aquatic sites where many eutrophication problems are attributable to phosphorus loading.

The Declaration of the Third North Sea Conference in 1990 specified that nutrient inputs entering areas of the marine environment that are, or are likely to become, eutrophic, must be reduced to 50% of their 1985 levels by 1995. The Fifth Conference in 2002 went further, aiming to eliminate eutrophication and create a healthy marine environment by 2010. Fine words.
The UK Environment Agency has developed a eutrophication strategy that promotes a coordinated framework for action, and a partnership approach at both national and local levels. The management of eutrophication requires targets and objectives to be agreed for different water bodies. Analysis of preserved plant and animal remains in sediments can be used to estimate the levels of nutrients that occurred in the past, when the water bodies concerned were less affected by eutrophication. These reference conditions can then be used to determine which waters are most at risk, or have already been damaged by eutrophication, and to prioritize sites for restorative action. The ability to measure and monitor levels of eutrophication has therefore become increasingly important.
4.1 Measuring and monitoring eutrophication
During the 1990s there was increased demand in the UK for effective methods of monitoring eutrophication. There was also considerable interest in the development of monitoring systems based on biotic indices. Several ‘quality indices’ based on a variety of organisms were explored. For monitoring tools to have practical application, they must satisfy certain requirements:
sampling must be quick and easy;
monitoring must be based on a finite number of easily identified groups; and
indices for evaluation must be straightforward to calculate.
Within-year variability in nutrient concentrations can be high, particularly for enriched waters. A high sampling frequency may therefore be required to provide representative annual mean data. In nutrient-enriched lakes, annual means are more likely to provide appropriate estimates of phosphorus than winter-spring means, due to the importance of internal cycling of nutrients in summer. This is an important consideration when designing sampling strategies for use in predictive models of trophic status.
The large group of algal species collectively known as diatoms has been used as indicators of eutrophication in European rivers. Individual species of diatom vary in their tolerance of nutrient enrichment, some species being able to increase their growth rates as nutrients become more available, whilst others are outcompeted and disappear. As diatoms derive their nutrients directly from the water column, and have generation times measured in days rather than months or years, the species composition of the diatom community should be a good indicator for assessing eutrophication. Convincing correlations have been demonstrated between aqueous nutrient concentrations and diatom community composition, but there are a number of other physical and chemical factors that also affect diatom distribution, such as water pH, salinity and temperature, which also need to be taken into account.
The UK Environment Agency has assessed the extent of eutrophication on the basis of concentrations of key nutrients (primarily nitrogen and phosphorus) in water, and the occurrence of obvious biological responses, such as algal blooms. There is an intention to rely more heavily in future on biological assessment schemes. One such system is based on surveys of the aquatic plant populations in rivers. Known as the mean trophic rank (MTR) approach, this uses a scoring system based on species and their recorded abundances at river sites. Each species is allocated a score (its species trophic rank, STR) dependent on its tolerance to eutrophication (Table 4.1); then, for a given site, the mean score for all species present is calculated. Tolerant species have a low score, so a low MTR tends to indicate a nutrient-rich river. In Britain, rivers in the north and west tend to have the highest MTR scores, whereas rivers in the south and east of England have the lowest. These scores reflect the influence of numerous factors, such as differences in river flow, patterns of agricultural intensification and variations in population density.
| Species | STR | Species | STR | Species | STR |
|---|---|---|---|---|---|
| Algae | Angiosperms | Angiosperms | |||
| Batrachospermum spp. | 6 | (a) Broadleaved species | (b) Grassleaved species | ||
| Hildenbrandia rivularis | 6 | Apium inundatum | 9 | Acorus calamus | 2 |
| Lemanea fluviatilis | 7 | A. nodiflorum | 4 | Alisma plantago-aquatica | 3 |
| Vaucheria spp. | 1 | Berula erecta | 5 | A. lanceolatum | 3 |
| Cladophora spp. | 1 | Callitriche hamulata | 9 | Butomus umbellatus | 5 |
| Enteromorpha spp. | 1 | C. obtusangula | 5 | Carex acuta | 5 |
| Hydrodictyum reticulatum | 3 | Ceratophyllum demersum | 2 | C. acutiformis | 3 |
| Stigeoclonium tenue | 1 | Hippurus vulgaris | 4 | C. riparia | 4 |
| Littorella uniflora | 8 | C. rostrata | 7 | ||
| Liverworts | Lotus pedunculatus | 8 | C. vesicaria | 6 | |
| Chiloscyphus polyanthos | 8 | Menyanthes trifoliata | 9 | Catabrosa aquatica | 5 |
| Jungermannia atrovirens | 8 | Montia fontana | 8 | Eleocharis palustris | 6 |
| Marsupella emarginata | 10 | Myriophyllum alterniflorum | 8 | Eleogiton fluitans | 10 |
| Nardia compressa | 10 | M. spicatum | 3 | Elodea canadensis | 5 |
| Pellia endiviifolia | 6 | Myriophyllum spp. Footnotes * | 6 | E. nuttallii | 3 |
| P. epiphylla | 7 | Nuphar lutea | 3 | Glyceria maxima | 3 |
| Scapania undulata | 9 | Nymphaea alba | 6 | Groenlandia densa | 3 |
| Nymphoides peltata | 2 | Hydrocharis morsus-ranae | 6 | ||
| Mosses | Oenanthe crocata | 7 | Iris pseudacorus | 5 | |
| Amblystegium fluviatilis | 5 | O. fluviatilis | 5 | Juncus bulbosus | 10 |
| A. riparium | 1 | Polygonum amphibium | 4 | Lemna gibba | 2 |
| Blindia acuta | 10 | Potentilla erecta | 9 | L. minor | 4 |
| Brachythecium plumosum | 9 | Ranunculus aquatilis | 5 | L. minuta/miniscula | 3 |
| B. rivulare | 8 | R. circinatus | 4 | L. trisulca | 4 |
| B. rutabulum | 3 | R. flammula | 7 | Phragmites australis | 4 |
| Bryum pseudotriquetrum | 9 | R. fluitans | 7 | Potamogeton alpinus | 7 |
| Calliergon cuspidatum | 8 | R. omiophyllus | 8 | P. berchtoldii | 4 |
| Cinclidotus fontinaloides | 5 | R. peltatus | 4 | P. crispus | 3 |
| Dichodontium flavescens | 9 | R. penicillatus pseudofluitans | 5 | P. friesii | 3 |
| D. pellucidum | 9 | R. penicillatus penicillatus | 6 | P. gramineus | 7 |
| Dicranella palustris | 10 | R. penicillatus vertumnus | 5 | P. lucens | 3 |
| Fontinalis antipyretica | 5 | R. trichophyllus | 6 | P. natans | 5 |
| F. squamosa | 8 | R. hederaceus | 6 | P. obtusifolia | 5 |
| Hygrohypnum luridum | 9 | R. sceleratus | 2 | P. pectinatus | 1 |
| H. ochraceum | 9 | Ranunculus spp. Footnotes * | 6 | P. perfoliatus | 4 |
| Hyocomium armoricum | 10 | Rorippa amphibia | 3 | P. polygonifolius | 10 |
| Philonotis fontana | 9 | R. nasturtium-aquaticum | 5 | P. praelongus | 6 |
| Polytrichum commune | 10 | Rumex hydrolapathum | 3 | P. pusillus | 4 |
| Racomitrium aciculare | 10 | Veronica anagallis-aquatica | 4 | P. trichoides | 2 |
| Rhynchostegium riparioides | 5 | V. catenata | 5 | Sagittaria sagittifolia | 3 |
| Sphagnum spp. | 10 | V. scutellata | 1 | Schoenoplectus lacustris | 3 |
| Thamnobryum alopecurum | 7 | Viola palustris | 9 | Scirpus maritimus | 3 |
| Sparganium emersum | 3 | ||||
| Fern-allies | S. erectum | 3 | |||
| Azolla filiculoides | 3 | Spirodela polyrhiza | 2 | ||
| Equisetum fluviatile | 5 | Typha latifolia | 2 | ||
| E. palustre | 5 | T. angustifolia | 2 | ||
| Zannichellia palustris | 2 |
Footnotes
Response to eutrophication: STR 1-3 most tolerant; STR 4-5 moderately tolerant; STR 6-7 moderately sensitive; STR 8-10 most sensitive. Back to main textFootnotes
* Average values for the genus are used when individual species cannot be identified. Back to main text4.2 Reducing eutrophication
In Britain, water supply companies have tended to regard eutrophication as a serious problem only when it becomes impossible to treat drinking water supplies in an economic way. Threshold concentrations at which action is taken to reduce nutrient loadings thus depend on economic factors, as well as wildlife conservation objectives.
There are two possible approaches to reducing eutrophication:
Reduce the source of nutrients (e.g. by phosphate stripping at sewage treatment works, reducing fertilizer inputs, introducing buffer strips of vegetation adjacent to water bodies to trap eroding soil particles).
Reduce the availability of nutrients currently in the system (e.g. by removing plant material, removing enriched sediments, chemical treatment of water).
4.3 Reducing the nutrient source
Europe is the continent that has suffered most from eutrophication, and increasing efforts are being made to restore European water bodies damaged by nutrient enrichment. If the ultimate goal is to restore sites where nature conservation interest has been damaged by eutrophication, techniques are required for reducing external loadings of nutrients into ecosystems.
Although algal production requires both nitrogen and phosphorus supplies, it is usually sufficient to reduce only one major nutrient. An analogy can be drawn with motor cars, which require lubricating oil, fuel and coolant to keep them moving and are likely to stop if they run short of any one of these, even if the other two are in plentiful supply. As phosphorus is the limiting nutrient in most freshwater systems, phosphorus has been the focus of particular attention in attempts to reduce inputs. In addition, nitrogen is less easily controlled: its compounds are highly soluble and can enter waterways from many diffuse sources. It can also be ‘fixed’ directly from the atmosphere. Phosphorus, on the other hand, is readily precipitated, usually enters water bodies from relatively few point sources (e.g. large livestock units or waste-water treatment works) and has no atmospheric reserve. However, efforts to reduce phosphorus loadings in some lakes have failed due to ongoing release of phosphorus from sediments. In situations where phosphorus has accumulated naturally (e.g. in areas with phosphate-rich rocks) and nitrogen increases have driven eutrophication, it may be necessary to control nitrogen instead.
4.4.1 Diversion of effluent
In some circumstances it may be possible to divert sewage effluent away from a water body in order to reduce nutrient loads. This was achieved at Lake Washington, near Seattle, USA, which is close to the sea. Lake Washington is surrounded by Seattle and its suburbs, and in 1955 a cyanobacterium, Oscitilloria rubescens, became dominant in the lake. The lake was receiving sewage effluent from about 70 000 people; this input represented about 56% of the total phosphorus load to the lake. The sewerage system was redesigned to divert effluent away from the lake, for discharge instead into the nearby sea inlet of Puget Sound. The transparency of the water in the lake, as measured by the depth at which a white disc could be seen, quickly increased from about 1 to 3 m, and chlorophyll concentrations decreased markedly as a result of reduced bacterial populations.
Diversion of effluent should be considered only if the effluent to be diverted does not constitute a major part of the water supply for the water body. Otherwise, residence times of water and nutrients will be increased and the benefits of diversion may be counteracted.
4.4.2 Phosphate stripping
It has been estimated that up to 45% of total phosphorus loadings to freshwater in the UK comes from sewage treatment works. This input can be reduced significantly (by 90% or more) by carrying out phosphate stripping. The effluent is run into a tank and dosed with a product known as a precipitant, which combines with phosphate in solution to create a solid, which then settles out and can be removed. It is possible to use aluminium salts as a precipitant, but the resulting sludge contains toxic aluminium compounds that preclude its secondary use as an agricultural fertilizer. There are no such problems with iron salts, so Fe(II) ammonium sulfate is frequently chosen as a precipitant. The chemicals required as precipitants constitute the major cost, rather than installations or infrastructure, and the process is very effective: up to 95% of the phosphate can be removed easily, and it is possible to remove more. Despite its effectiveness, however, phosphate stripping is not yet used universally in sewage treatment.
4.4.3 Buffer strips
The interface between aquatic ecosystems and the land is an ecotone that has a profound influence on the movement of water and water-borne contaminants. Vegetation adjacent to streams and water bodies can help to safeguard water quality, particularly in agricultural landscapes. Buffer strips are used to reduce the amounts of nutrients reaching water bodies from runoff or leaching. They usually take the form of vegetated strips of land alongside water bodies: grassland, woodland and wetlands have been shown to be effective in different situations. The vegetation often performs a dual role, by reducing nutrient inputs to aquatic habitat and also providing wildlife habitat. A riparian buffer zone of between 20 and 30 m width can remove up to 100% of incoming nitrate. The plants take up nitrogen directly, provide a source of carbon for denitrifying bacteria and also create oxidized rhizospheres where denitrification can occur. Uptake of nitrogen by vegetation is often seasonal and is usually greater in forested areas with sub-surface water flow than in grassland with predominantly surface flow. The balance between surface flow and sub-surface flow, and the redox conditions that result, are critical in determining rates of nitrate removal in buffer strips (Figure 4.4).
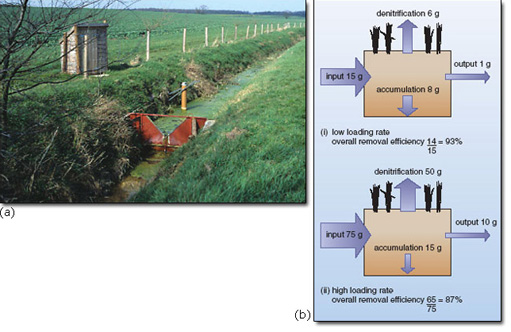
The dynamics of nitrogen and phosphorus retention by soil and vegetation can alter during succession. In newly constructed wetlands, nitrogen retention commences as soon as emergent vegetation becomes established and soil organic matter starts to accumulate: usually within the first 1-3 years. Accumulation of organic carbon in the soil sets the stage for denitrification. After approximately 5-10 years, denitrification removes approximately the same amount of nitrogen as accumulates in organic matter (about 5-10 gm−2 yr−1 under conditions of low nitrogen loading). Under higher nitrogen loading, the amount of nitrogen stored in accumulating organic matter may double, and nitrogen removal by denitrification may increase by an order of magnitude or more. Accumulation of organic nitrogen and denitrification can therefore provide for reliable long-term removal of nitrogen regardless of nitrogen loading.
Phosphorus removal, on the other hand, tends to be greater during the first 1-3 years of succession when sediment deposition and sorption (absorption and adsorption) and precipitation of phosphorus are greatest. During the early stages of succession, wetlands may retain from 3 g P m−2 yr−1 under low phosphorus loadings, and as much as 30 g P m−2 yr−1 under high loadings. However, as sedimentation decreases and sorption sites become saturated, further phosphorus retention relies upon either its accumulation as organic phosphate in plants and their litter, or the precipitation with incoming aqueous and particulate cations such as iron, aluminium and calcium.
Nevertheless, in general, retention of phosphorus tends to be largely regulated by geochemical processes (sorption and precipitation) which operate independently of succession, whereas retention of nitrogen is more likely to be controlled by biological processes (e.g. organic matter accumulation, denitrification) that change in relative significance as succession proceeds.
Surface retention of sediment by vegetated buffer strips is a function of slope length and gradient, vegetation density and flow rates. Construction of effective buffer strips therefore requires detailed knowledge of an area’s hydrology and ecology. Overall, restoration of riparian zones in order to improve water quality may have greater economic benefits than allocation of the same land to cultivation of crops.
4.3 Reducing the nutrient source, continued
4.3.4 Wetlands
Wetlands can be used in a similar way to buffer strips as a pollution control mechanism. They often present a relatively cost-effective and practical option for treatment, particularly in environmentally sensitive areas where large waste-water treatment plants are not acceptable. For example, Lake Manzala in Egypt has been suffering from severe pollution problems for several years. This lake is located on the northeastern edge of the Nile Delta, between Damietta and Port Said. Land reclamation projects have reduced the size of the lake from an estimated 1698 km2 to 770 km2. The lake is shallow, with an average depth of around 1.3 m.
Five major surface water drains discharge polluted waters into the lake. These waters contain municipal, industrial and agricultural pollutants, which are causing water quality to deteriorate and fish stocks to decline. Recently, efforts have been made to improve water quality in the most polluted of the five drains. This carries waste water from numerous sources, including sewage effluent from Cairo, waste water from industries, agricultural discharges from farms, and discharges and spills from boat traffic. Several methods for drain water treatment have been proposed, including conventional waste-water treatment plants and other chemical and mechanical methods for aerating the drain water. There are also proposals for construction of a wetland to treat approximately 25 000 m3 per day of drain water and discharge the treated effluent back to the drain.
The treatment process involves passing the drain water through basins and ponds, designed to have specific retention times. The pumped water first passes through sedimentation basins to allow suspended solids to settle out (primary treatment), followed by a number of wetland ponds (secondary treatment). The ponds are cultivated with different types of aquatic plants, such as emergent macrophytes (e.g. Phragmites) with well-developed aerenchyma systems to oxygenate the rhizosphere, allowing the oxidation of ammonium ions to nitrate. Subsequent denitrification removes the nitrogen to the atmosphere.
The waste-water treatment mechanism depends on a wide diversity of highly productive organisms, which produce the biological activity required for treatment. These include decomposers (bacteria and fungi), which break down particulate and dissolved organic material into carbon dioxide and water, and aquatic plants. Some of the latter are able to convey atmospheric oxygen to submerged roots and stems, and some of this oxygen is available to microbial decomposers. Aquatic plants also sequester nitrogen and phosphorus. Species such as common reed (Phragmites australis, Figure 4.5) yield a large quantity of biomass, which has a range of commercial uses in the region. Another highly productive species is the water hyacinth (Eichhornia crassipes, Figure 4.6). This species is regarded as a serious weed on the lake and is regularly harvested to reduce eutrophication. However, it has a potential role in water treatment due to its high productivity and rapid rates of growth. The resultant biomass could possibly be harvested and used for the production of nutrient-rich animal feed, or for composting and the production of fertilizer. Further research is required to develop practical options.


The passage of water through emergent plants reduces turbidity because the large surface area of stems and leaves acts as a filter for particulate matter. Transmission of light through the water column is improved, enhancing photosynthesis in attached algae. These contribute further to nutrient reduction in through-flowing water. The mixture of floating plants and emergent macrophytes contributes to removal of suspended solids, improved light penetration, increased photosynthesis and the removal of toxic chemicals and heavy metals.
Estimates for the removal of total suspended solids (TSS), biological oxygen demand (BOD), total phosphorus and total nitrogen by the different wetland components are provided in Table 4.2. These suggest that wetlands, combined with sedimentation and ancillary water treatment systems, could play an important part in reducing nutrient loadings.
| Parameter | Sedimentation pond | Wetland treatment system | ||||
|---|---|---|---|---|---|---|
| influent conc./mg l−1 | effluent conc./mg l−1 | removal efficiency/% | influent conc./mg l−1 | effluent conc./mg l−1 | removal efficiency/% | |
| TSS | 160 | 32 | 80 | 32 | 6.4 | 80 |
| BOD | 40 | 24 | 40 | 24 | 18.0 | 25 |
| total P | 5 | 4 | 20 | 4 | 3.2 | 20 |
| total N | 12 | 12 | 0 | 12 | 8.4 | 30 |
| organic N | 4 | 4 | 0 | 4 | 3.8 | 5 |
| ammonium N | 5 | 5 | 0 | 5 | 3.9 | 22 |
4.3.5 Domestic campaigns
An important aspect of efforts to reduce nutrient inputs to water bodies is the modification of domestic behaviour. Public campaigns in Australia have encouraged people to:
wash vehicles on porous surfaces away from drains or gutters
reduce use of fertilizers on lawns and gardens
compost garden and food waste
use zero- or low-phosphorus detergents
wash only full loads in washing machines
collect and bury pet faeces.
These campaigns have combined local lobbying with national strategies to tackle pollution from other sources.
4.4 Reducing nutrient availability
Once nutrients are in an ecosystem, it is usually much harder and more expensive to remove them than tackle the eutrophication at source. The main methods available are:
precipitation (e.g. treatment with a solution of aluminium or ferrous salt to precipitate phosphates);
removal of nutrient-enriched sediments, for example by mud pumping; and
removal of biomass (e.g. harvesting of common reed) and using it for thatching or fuel.
In severe cases of eutrophication, efforts have been made to remove nutrient-enriched sediments from lakes. Lake Trummen in Sweden accumulated thick black sulfurous mud after years of receiving sewage effluent. Even when external loadings of phosphorus were reduced to 3 kg P yr−1, there was still an internal load (i.e. that derived from the lake’s own sediment) of 177 kg P yr−1! Drastic action was needed. Eventually nutrient-rich sediment was sucked from the lake and used as fertilizer. The water that was extracted with the sediment was treated with aluminium salts and run back into the lake. This action reduced phosphorus concentrations and improved the clarity and oxygenation of the water. However, removal or sealing of sediments is an expensive measure, and is only a sensible option in severely polluted systems, such as the Norfolk Broads, England.
Removal of fish can allow species of primary consumers, such as the water-flea, Daphnia, to recover and control algae. Once water quality has improved, fish can be re-introduced.
Mechanical removal of plants from aquatic systems is a common method for mitigating the effects of eutrophication (Figure 4.7). Efforts may be focused on removal of existing aquatic ‘weeds’ such as water hyacinth that tend to colonize eutrophic water. Each tonne of wet biomass harvested removes approximately 3 kg N and 0.2 kg P from the system.

Alternatively plants may be introduced deliberately to ‘mop up’ excess nutrients. Although water hyacinth can be used in water treatment, the water that results from treatment solely with floating macrophytes tends to have low dissolved oxygen. Addition of submerged macrophytes, together with floating or emergent macrophytes, usually gives better results. Submerged plants are not always as efficient as floating ones at assimilating nitrogen and phosphorus due to their slower growth, resulting from poor light transmission through water (particularly if it is turbid) and slow rates of CO2 diffusion down through the water column. However, many submerged macrophytes have a high capacity to elevate pH and dissolved oxygen, and this improves conditions for other mechanisms of nutrient removal. At higher pH, for example, soluble phosphates can precipitate with calcium, forming insoluble calcium phosphates, so removing soluble phosphates from water. Various species have been used in this way. One submersed macrophyte, Elodea densa, has been shown to remove nitrogen and phosphorus from nutrient-enriched water, its efficiency varying according to loading rate. Nitrogen removal rates reached 400 mg N m−2 per day during summer, while for phosphorus over 200 mg P m−2 per day were removed.
In terrestrial habitats, removal of standing biomass is an important tool in nature conservation. Reduction in the nutrient status of soils is often a prerequisite for re-establishment of semi-natural vegetation, and the removal of harvested vegetation helps to reduce the levels of nutrients returned to the soil (Figure 4.8). However, if the aim is to lower the nutrient status of a nutrient-enriched soil, this can be a very long-term process (Figure 4.9).

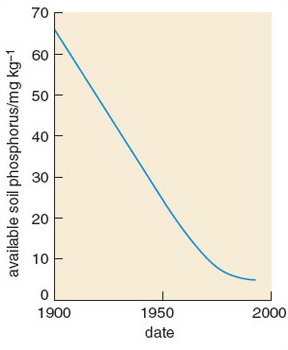
Question 4.1
A short reach of the River Great Ouse in Bedfordshire was found to contain the following species:
| Common name | Scientific name |
|---|---|
| filamentous alga | Cladophora spp. |
| moss | Amblystegium riparium |
| fool’s water-cress | Apium nodiflorum |
| hornwort | Ceratophyllum demersum |
| mare’s-tail | Hippuris vulgaris |
| spikedwater-milfoil | Myriophyllum spicatum |
| yellow water-lily | Nuphar lutea |
| great water dock | Rumex hydrolapatham |
| water speedwell | Veronica anagallis-aquatica |
| sweet flag | Acorus calamus |
| water plantain | Alisma plantago-aquatica |
| lesser pond sedge | Carex acutiformis |
| reed sweet-grass | Glyceria maxima |
| yellow flag iris | Iris pseudacorus |
| greater duckweed | Lemna gibba |
| broadleaved pondweed | Potamogeton natans |
| bulrush | Schoenoplectus lacustris |
A similar length of the River Eden in Cumbria was found to have the following species:
| Common name | Scientific name |
|---|---|
| liverwort | Pellia epiphylla |
| moss | Calliergon cuspidatum |
| river moss | Fontanalis antipyretica |
| water horsetail | Equisetum fluviatile |
| white water-lily | Nymphaea alba |
| lesser spearwort | Ranunculus flammula |
| pinkwater speedwell | Veronica catenata |
| sedge | Carex acuta |
| bottle sedge | Carex rostrata |
| common spike-rush | Eleocharis palustris |
| broadleaved pondweed | Potamogeton natans |
Using the trophic rank scores in Table 4.1, calculate the mean trophic rank (MTR) for each stretch of river, and comment on whether the watercourse is nutrient-enriched (eutrophic). Assume all the species recorded are of similar abundance and therefore there is no need to weight scores according to relative abundance, as you would do in a real situation.
Answer
The River Great Ouse has a MTR of 3, suggesting it is enriched with nutrients and therefore eutrophic, but it is on the mildest edge of this category so the eutrophication is not severe.
The River Eden has an MTR of 6, indicating that its plant community is composed of species that are moderately sensitive to enrichment, so it can be assumed that this stretch has not undergone substantial eutrophication. The most sensitive species are absent, suggesting that the waters may naturally carry a moderate concentration of nutrients or that some very mild enrichment has occurred.
Question 4.2
List the advantages of preventing eutrophication at source, compared with treating its effects.
Answer
Prevention of eutrophication at source compared with treating its effects (or reversing the process) has the following advantages.
Technical feasibility. In some situations prevention at source may be simply engineered by diverting a polluted watercourse away from the sensitive ecosystem, while removal of nutrients from a system by techniques such as mud-pumping is more of a technical challenge.
Cost. Nutrient stripping at source using a precipitant is relatively cheap and simple to implement. Biomass stripping of affected water is labour-intensive and therefore expensive.
Habitat availability. Buffer strips and wetlands can provide a stable wildlife habitat whilst performing a nutrient trapping role on throughflow water. Habitats, whether aquatic or terrestrial, can be compromised in terms of their wildlife value, due to the degree of disturbance involved in biomass stripping.
Products. Constructed wetlands may be managed to provide economic products such as fuel, compost or thatching material more easily than trying to use the biomass stripped from a less managed system.
Conclusion
Eutrophication is a process in which an ecosystem accumulates mineral nutrients. It can occur naturally, but is usually associated with human activity that releases nutrients into the environment.
Anthropogenic eutrophication has caused a widespread loss of biodiversity in many systems. Recent attempts to reverse the process are proving difficult and expensive.
Symptoms of eutrophication are most readily seen in aquatic systems, where the additional nutrients lead to the explosive growth of algal or bacterial populations. The large biomass produced excludes light from the water and can result in the deoxygenation of the water, killing fish and other animals.
In terrestrial systems, additional nutrients boost the productivity of competitive plant species. These then exclude less competitive species by shading them, leading to a decrease in species richness. The humped-back curve describes the relationship between biomass and species richness.
Estuaries are particularly prone to eutrophication, and like other aquatic environments can suffer from algal blooms that eliminate other species. Loss of key species, such as seagrass, results in an entire habitat type and all its dependent species disappearing.
The main agents of eutrophication are compounds containing the elements phosphorus and nitrogen. It is these elements that, under natural conditions, usually limit the primary production in ecosystems. Increasing their supply therefore increases productivity.
Sources of anthropogenic phosphorus entering the environment include sewage discharges, intensive livestock farms and the spreading of artificial fertilizers and animal manures onto agricultural land. The majority of phosphorus comes from point sources.
Sources of anthropogenic nitrogen entering the environment include gaseous emissions from car exhausts and power stations and artificial fertilizers applied to agricultural land. The majority of nitrogen comes from diffuse sources.
Recent European legislation has tried to limit further eutrophication of the environment by measures such as the stripping of phosphorus from waste water and the control of nitrogen fertilizer applications in sensitive zones.
Living organisms can be used as monitors of the trophic status of ecosystems.
Removal of nutrients from an ecosystem in order to reverse the effects of eutrophication is a difficult and expensive undertaking.
Acknowledgements
Course image: Nic Redhead in Flickr made available under Creative Commons Attribution-NonCommercial-ShareAlike 2.0 Licence.
The content acknowledged below is Proprietary (see terms and conditions). This content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence
Grateful acknowledgement is made to the following sources for permission to reproduce material in this unit:
Figures 1.1, 1.2, 1.3, 1.10, 2.4, 2.8, 2.10, 2.11, 2.11, 2.17, 3.2, 3.3, 4.2, 4.3 & 4.7 Courtesy of the Environment Agency;
Figures 1.4, 1.8a, b, 2.3, 2.12, 2.14, 2.15, 2.16, 3.1, 4.8 Copyright © Mike Dodd/The Open University;
Figure 1.7 Andy Harmer/Science Photo Library;
Figure 1.8c Courtesy of Dr John Madsen/Minnesota State University;
Figure 1.12 Copyright Dr Julian Thompson, Wetland Research Unit, Department of Geography, University College, London;
Figure 2.1 Copyright © Sukurta Viliaus & Co.;
Figures 2.2, 2.5a & 3.4 Copyright © Owen Mountford/Centre for Ecology and Hydrology;
Figure 2.5b Copyright © Peter Gathercole/Oxford Scientific Films;
Figure 2.6 Copyright © Alistair MacEwen/Oxford Scientific Films;
Figure 2.7 Copyright © BBC Worldwide;
Figure 2.18 With permission from Professor Chris Kjeldsen, Biology Department, Sonoma State University;
Figure 2.24 Graham Day;
Figure 4.4 Copyright © Peter Leeds-Harrison/Cranfield University;
Figure 4.5 Copyright © ARM Limited. Reproduced with permission of ARM Limited, Rydal House, Colton Road, Rugeley, Staffordshire, WS15 3HF, UK, armreedbeds.co.uk, designers of reed beds and welands for wastewater treatment;
Figure 4.6 Pisces Conservation Limited.
Courtesy of the Environment Agency;
Don’t miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
Copyright © 2016 The Open University