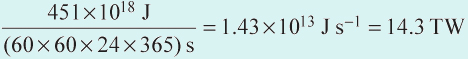Energy resources: An introduction to energy resources
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Saturday, 20 April 2024, 4:18 AM
Energy resources: An introduction to energy resources
An introduction to energy resources
Understanding energy resources involves considering all types of energy source from various scientific and technological standpoints, with a focus on the uses, limitations and consequences of using energy that is available to humanity. This course sets the scene by considering how much energy human society uses and the basic concepts of energy, work, power and efficiency, then briefly investigates the different types of energy available, their sources and renewability.
This OpenLearn course provides a sample of level 2 study in Science
Learning outcomes
After studying this course, you should be able to:
understand the difference between energy and power, and their units and prefixes
state the relative contributions of different natural energy sources to the global energy budget
describe the contribution of photosynthesis to the carbon cycle, and distinguish the terrestrial and marine parts of the cycle
discuss the issues involved in concentrating, storing and transporting energy
recognise which energy resources have a low energy density, and which have a high energy density.
1 Energy use
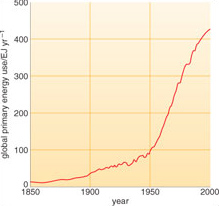
Until about 8000 years ago humans relied on hunting and gathering for food, and burning wood to keep warm. Their exact energy demands can at best only be estimated but to survive they probably needed about as much energy as it takes to run a couple of ordinary domestic light bulbs continuously. Later, agriculture developed, and although wood was still the chief fuel, animal power, animal dung and charcoal were also used. Even today, such energy sources based on natural biomass dominate the lives of human populations in the so-called 'Third World' or 'developing countries'. The 19th century heralded a large increase in energy use in what were to become industrialised countries (Figure 1.1), particularly the use of coal. Homes and other buildings were heated; factories and railways were powered by steam engines (Figure 1.2); mining and chemical industries developed and agriculture became more mechanised. The emergence of technological societies in the 20th century resulted in an even larger increase in energy use for manufacturing, agriculture, transport and a host of other applications. In technologically advanced countries the largest increases have been in using gas for heating, oil products for transport, and electricity as a convenient means of transferring energy generated by a variety of sources (Figure 1.3).

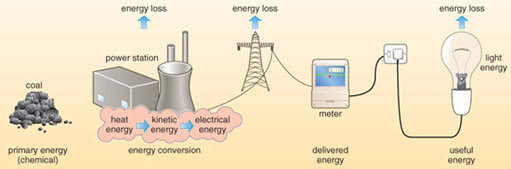
It is important to remember that the primary energy released by all forms of energy resources is not the amount that performs useful tasks: it is the total amount of energy released by human activity. Energy use and conversion, as you will see, can never be fully efficient. For that reason, the energy consumed usefully by society is considerably less than primary energy released: you will find that we refer to both primary energy and energy consumption (sometimes demand), depending on the context.
World population rose from some 5 million 10 000 years ago, through 1 billion in the 19th century to 6.5 billion in 2005. This rapid increase in population, together with a sharp increase in the demand that each person in the developed world has for energy, led to the dramatic rise in global energy consumption (Figure 1.1).
All the Earth's physical resources, for example metals in ores, water supplies and building stone, depend on using energy to extract, process and transport them. In effect, the ability to extract and use the Earth's physical resources depends on whether there is a ready supply of energy at the right price. If there were a limitless supply of cheap energy we could turn the entire stock of all physical resources into reserves. One aim of this book, and indeed the whole course, is to examine the limits that exist in reality: some are governed by physical laws, others depend on economics and there are also limits posed by sustaining the Earth's environmental conditions on which life depends.
2 Energy, work, power and efficiency
In everyday speech we often refer colloquially to the powerful politician, the energetic child, the working mother and the efficient administrator. We use these terms imprecisely, and often wrongly, compared with their scientific definitions.
2.1 Some basic concepts
Energy is defined as the capacity to do work, and work is even more precisely defined as a force acting on an object that causes its displacement, and is calculated from force × distance. Work is therefore the foundation for scientific study of motion and change.
The unit of work is the same as that for energy: the joule (J). Yet a joule, unlike the units of mass, length or time, is not a fundamental unit. Working out the joule in fundamental units takes us to the root of the physics involved. The definition of work (force × distance) shows that one joule is actually one newton metre (N m).
Force is mass × acceleration, so one newton is the force that gives a mass of one kilogram (kg) an acceleration of one metre per second per second (m s−2), and is therefore equivalent to 1 kg m s−2.
However, work is not just mechanical movement, such as moving sacks of flour around or turning a wheel. It is also involved in heating a substance (vibrating its molecules), changing its state (melting and boiling) and compressing it, along with many other phenomena.
As an example of the connection between work and energy consider the energy bound up with a photon of light that strikes the photoelectric cell in a solarpowered pocket calculator. Some of that photon's energy becomes an electrical current that contributes to the calculator's microchip executing a calculation. However, most of the original light energy goes to heating up the cell and the liquid crystal display that shows the answer, and there might even be a beep of sound when the calculation is complete. During this process a tiny amount of work is done, but the form of the energy has changed, from the otherwise limitless potential of light to travel an infinite distance through a vacuum, never losing its 'capacity to do work', to the motion of particles of matter. The last change is eventually expressed by a minuscule rise in temperature in and around the calculator, which ultimately dissipates to help heat up the rest of the Universe! Energy can neither be created or destroyed (the Law of Conservation of Energy) so the energy of the photon doesn't disappear but is spread far and wide. Although eternal, in practice it is beyond recovery: useful energy in a high grade form is degraded through the material work that it has done.
Energy takes many natural forms: light, heat, sound, mechanical movement (kinetic energy), that gained by position in a gravitational field (potential energy), the movement of electrons (electricity), chemical energy, that released through Einstein's famous matter-energy conversion E = mc2 (nuclear energy) and a great many more. In this book we deal mainly with the forms of energy that are available at sufficiently high grade to be able to do useful work. An implicit theme is that all energy sources are themselves products of work done naturally:
Chemical energy — 'fossil fuels', such as coal and petroleum, and 'biofuels', such as wood, are the products of conversion of solar energy into the energy of chemical combination through photosynthesis by plants;
Nuclear energy — nuclear fuels are heavy radioactive isotopes produced by thermonuclear fusion in long-dead stars that became supernovae;
Geothermal energy comes from heat produced by the natural decay of radioactive isotopes distributed at very low concentrations in the Earth;
Solar energy is emitted by thermonuclear fusion within the Sun;
Tidal energy is essentially the work done by the gravitational fields of the Moon and Sun on the oceans;
Wind and wave energy are solar energy converted into work done by the Earth's atmosphere and oceans;
Hydro energy relies on work done by solar energy in evaporating water that falls as rain or snow at high topographic elevations, which in turn has gravitational potential energy.
Every application that 'uses' energy is in fact converting one form of energy into other forms. Some of this energy conversion is useful, some is not. A kettle boils water, but it may also 'sing' (sound energy) and heat up itself. A hydroelectric power station turns the kinetic energy of moving water into electricity through generators, but at the same time friction between the moving parts of the generators produces heat and sound. The heat and sound are degraded and less useful forms of energy.
Question 1
Which energy changes take place when an electric kettle boils water?
Answer
Electrical energy is first converted into heat energy as it increases the vibrational movement of atoms in the metal heating element. Heat is conducted through the element into the water, making the water molecules vibrate more, thereby raising its temperature. Finally, heat energy converts water into steam.
The point to be grasped here is that changes of energy from one form to another are commonplace in everyday life.
Power is the rate at which energy is delivered or work is done. It is worth noting the difference between power and energy: energy is an amount of work done over an indefinite time interval and power is the rate at which energy is converted, i.e. the amount used or made available per second. The units for measuring energy and power are summarised in Box 1.1 below.
Box 1.1 Units of energy and power
All forms of energy are measured in the same unit, the joule (J). A joule is the same amount of energy irrespective of which form (light, sound, electrical, and so on) that energy takes.
Recalling the earlier definitions in this section, when 1 kg falls 1 m at the acceleration due to gravity at the Earth's surface (9.81 m s−2), the work done (force × distance) is mass in kg × acceleration due to gravity in m s−2 × height in m, i.e.:
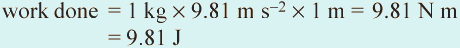
So a joule is equivalent to the work done when a mass of (1/9.81) kg or 0.102 kg falls through a metre: a mass about the size of the apple reputed to have fallen on Isaac Newton's head to inspire his theory of gravity! A joule is a small amount of energy so when considering national or international energy use, large multiples of the joule are needed, and these are named in the metric (SI) system using standard prefixes (Table 1.1).
There is another energy unit that will be used occasionally in this book, the tonne oil equivalent (toe). This is often used by energy statisticians as a convenient means of comparing amounts of energy available from fuels other than oil. One toe is the chemical energy contained in one tonne of oil, and equates approximately to 4.19 × 1010 J (i.e. 41.9 GJ).
Being a rate, power is measured in joules converted per second (J s−1), called watts (W). One watt is equivalent to one joule per second. Note that if you are hit on the head by a falling apple, even though its energy is only about 1 J, the impact is almost instantaneous, so its power is high. Just as a joule is a small unit of energy, so a watt is a small unit of power. Larger quantities of power are quoted using the same prefixes as energy, as given in Table 1.1.
An electric supply of 1 kW will run a microwave oven, or around 10 light bulbs. Working really hard, the human body can deliver about 1 kW for a very short period of time.
Every three months or so most people in the UK receive an electricity bill, which states the number of 'Units' of electricity they have used, as measured by their electricity meter. The 'Units' quoted are kilowatt hours (kW h). But what, in purely physical terms, does 1 kW h represent? One kilowatt hour is the energy delivered at a power of 1 kW for one hour. This is equivalent to 1 kJ per second for an hour, i.e. 3600 kJ.
In practical terms, you might think that one kW h was a measure of all the work done around your home by various appliances in an hour. You would be very disappointed, because not one of them is perfectly efficient. In fact over 70% of each 'Unit' would ultimately have been lost to heating the rest of the Universe without doing any useful work. As you will see below, generating and then transmitting the electrical energy to your home will have been inefficient too, so a high proportion of your bill had been spent on work that was of no practical use to you.
When we convert energy from one form to another, the output that is useful to us is never as much as the energy input. The ratio of the useful output to the input (i.e. the ratio of useful work done to the energy supplied) is called the energy efficiency of the process and is usually expressed as a percentage. Every natural process involves a change in grade of energy through the work that it does, but one joule always ends up as one joule, because of the Law of Conservation of Energy. Ignoring this transformation in grade, energy conversion could be said to be 100% efficient. But that misses the point. A perfect energychanging machine would get out as much useful energy as was put in, so it would be 100% efficient, but such a machine does not and probably cannot exist.
Efficiency can be as high as 90% in a water turbine, but only around 35-40% in a coal-fired power station. The energy delivered to do useful work is considerably less than the primary energy consumed in electricity generation.
2.2 Present-day energy use
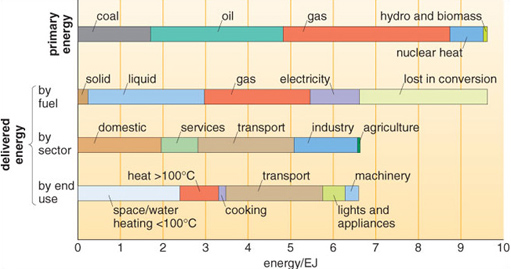
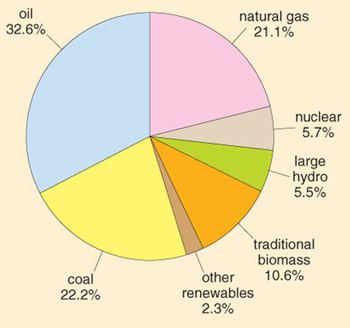
Global annual consumption of all forms of primary energy increased more than tenfold during the 20th century (Figure 1.1), and by the year 2002 reached an estimated 451 EJ. About three-quarters of this energy came from coal, oil and gas (Figure 1.4).
Make a note of these equivalent amounts, as you will be comparing them with the energy and power available from various sources later.
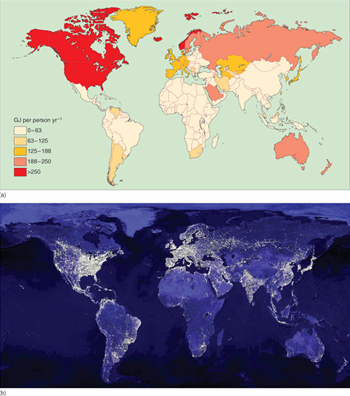
With a global population of 6.5 billion, each person's 'drain' on primary energy is, on average, around 73 GJ per year. But globally, there are major regional differences in energy consumption (Figure 1.5a). Developed countries, with industrial as well as domestic demands, use energy in vast quantities and at alarming rates. In North America it is around 350 GJ per person per year, nearly five times the global average, and totalling around 28% of global energy use by about 4.5% of world population. People in Europe and the former Soviet Union use about double the global average. Figure 1.5b, which shows the amount of lighting seen from space at night, gives a graphic picture of the inequalities of energy use.
In 2002, UK primary energy used was the equivalent of 9.7 EJ: about 164 GJ for each of the 59 million people in the UK, just over double the global average. About one-fifth of the UK's primary energy requirement is used in the home, 30% lost in conversion and most of the rest for services, transport and industry (Figure 1.6 below).
2.3 Global power demand
In Section 2.2 we calculated a value of 14.3 TW for the average global requirement for primary power in 2002.
Question 3
The average global figure of 2.3 kW per person is about five times less than that needed to enable each North American citizen to sustain the lifestyle to which he or she has grown accustomed. If every individual in the world were to demand as much energy as the average person uses in North America, the global energy supply industries would require a fivefold increase in their use of primary energy sources. Even more daunting is the prospect of continued growth of both world population and per capita energy demand.
3 Sources of energy from the natural environment
The natural environment itself is bathed in energy from other sources. Standing on a cliff top on a bright spring day you can feel the warmth of the Sun and the freshness of the breeze and hear the crashing of breaking waves below. All these energetic processes can be compared in terms of energy and power.
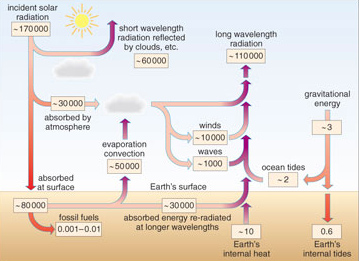
In order to put the total global energy supply and demand into proper perspective we need to know the contribution made by different natural energy sources to the Earth's energy supply. By far the most important source on Earth is the Sun, but some energy comes from the gravitational attraction between Sun, Moon and Earth, and some from the Earth's own internal heat. Figure 1.7 gives all the data on natural energy sources you will need in working through this section.
3.1 Solar radiation
Over 99.9% of the energy available at the Earth's surface comes from the Sun. Solar energy emanates from a vast nuclear powerhouse producing heat, light and other types of electromagnetic radiation released by nuclear reactions. The Sun's power output is enormous, some 1014 TW, but only a tiny proportion, about 1.7 × 105 TW, reaches the Earth. About a third of this is reflected by clouds and the Earth's surface directly back into space (Figure 1.7).
Question 4
How much energy reaches the Earth (surface and atmosphere together) from the Sun each year? (Don't forget the numbers in Figure 1.7 are in terms of power.)
Answer
The Sun supplies 1.7 × 105TW to the Earth, of which 1.1 × 105TW enters the Earth's system (0.3 × 105TW to atmospheric heating + 0.8 × 105TW absorbed at the surface, see Figure 1.7). 1.1 × 105TW is equivalent to 1.1 × 1017 J s−1, and since there are 3.15 × 107 s in a year, the Sun's annual energy supply is 3.15 × 107 s × 1.1 × 1017 J s−1 = 3.5 × 1024 J or 3.5 × 106 EJ.
If all the solar energy that reaches the Earth could be harnessed, current human needs would be supplied thousands of times over. The reason it cannot is explained later.
Solar radiation is potentially available as an energy resource either as direct solar energy using solar cells or heating devices (Figure 1.8), or naturally through plant and animal growth. Some 50 000 TW of absorbed solar radiation is transferred back into the atmosphere through evaporation and convection (Figure 1.7). Some of this energy reappears in a usable form when water eventually returns to the surface as precipitation. This energy is largely dissipated as frictional heat and sound during the return flow of the water to the oceans, but it can also be harnessed as hydropower, an indirect form of solar energy.

However, if the electrical power requirement of an average British household — around 3 kW — came from solar energy alone, even if every day were sunny each dwelling in Britain would need about 100 m2 of solar panels, on a very large south-facing roof. But is solar energy the answer to energy supply in sunnier parts of the world?
Question 5
California has an area of about 4.1 × 1011 m2. Even if the Sun shines there for 12 hours each and every day of the year, what percentage of California's land surface area would need to be covered by solar panels to supply the energy demands of the whole of the USA? The USA used 96.5 EJ of energy in 2003. A 10% efficient, metre-square solar panel would supply 31 J every second.
Answer
Assuming that solar panels operated for 1.6 × 107 seconds a year (12 hours a day), one such panel would supply about 5 × 108 J. The USA would therefore need around 2.0 × 1011 such panels (i.e. 96.5 × 1018 J divided by 5 × 108 J). Since the area of California is 4.1 × 1011 m2, about 50% of it would have to be covered by solar panels to meet US energy demand. (The assumptions we have made are so unrealistic that the figure would be much higher, were a 'solar energy only' scheme to be implemented.)
What happens to the solar energy that reaches the Earth's surface? Because the Earth is a sphere, the heating effect of the Sun is greater in equatorial latitudes than at the poles. Coupled with the Earth's rotation, this produces winds which blow between belts of high and low atmospheric pressure. About 10% of the Earth's absorbed solar power (10 000 TW) gives rise to winds (Figure 1.7). Much of the energy of winds is dissipated as heat, but some 10% of this wind power (i.e. 1000 TW) is transferred to waves through frictional effects at the sea surface.
Question 6
Could the world be supplied from wind and wave power one day?
Answer
Theoretically, yes, but practically, no. Total global wind power amounts to 10 000 TW, i.e. around one thousand times human power demands. However, tapping wind power over one-thousandth of the world's surface (some 510 000 km2 or over twice the size of Britain) would be a colossal undertaking and the process would have to be fully efficient. Similar arguments apply to wave power, except that here a maximum of 1000 TW is theoretically available (100 times annual demand), requiring twice the surface area of the Mediterranean Sea to be tapped at 100% efficiency.
3.2 Tides
Tides are caused by the gravitational pull of the Moon and to a lesser extent the Sun. Although tides affect all fluid bodies on Earth in some measure, including some parts of the solid Earth itself, their main effect is on the seas and oceans. Ultimately the kinetic energy of tides is converted into heat, mainly through friction between water and the sea bed. Tides can be exploited as an energy resource, and the total amount of power available can be calculated from knowledge of the gravitational effects of the Earth-Moon-Sun system. At about 2.7 TW, it is many orders of magnitude less than the power of solar radiation (Figure 1.7) and less than 20% of the current power demand for human activities.
3.3 The Earth's internal heat
The occurrence of both volcanoes and hot springs shows that the Earth's interior is hot, producing molten rock at temperatures up to 1250 °C, and also superheated steam. However, these phenomena are mainly confined to several narrow zones along the world's active plate boundaries. Many measurements have now been made of the amount of heat flowing from the Earth's interior. Outside the distinctive zones mentioned above, heat flow varies from 40-120 milliwatts per square metre (mW m−2), largely generated by the decay of long-lived radioactive isotopes within the Earth. The total power output from the Earth's interior, estimated at some 10 TW, is many orders of magnitude less than the total incident solar power (Figure 1.7).
Question 7
4 Fossil fuels
Part of the incoming solar energy becomes stored in fossil fuels (oil, gas and coal: Figure 1.7). To understand why fossil fuels exist you need first to know where the major stores of carbon are on the planet and how, through organic activity, this carbon becomes fixed in rocks and thus liable to be stored for geologically long periods.
4.1 Natural stores of carbon
The major natural stores of carbon (called 'reservoirs') are shown below in Figure 1.9.
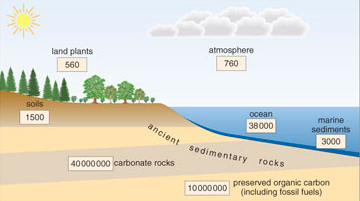
Question 8
Which two reservoirs contain most of the carbon?
Answer
Carbonate rocks and preserved organic carbon.
Carbon is being exchanged continually between the principal reservoirs shown in Figure 1.9. However, since most stored carbon is held in carbonate rocks and preserved organic carbon (POC), the principal carbon exchange over geological timescales (millions of years) is from the surface reservoirs into limestones and POC.
For our purposes two exchange systems can be distinguished (somewhat artificially because in practice the two are intimately linked):
the land-based or terrestrial system in which carbon is exchanged between land plants and both the soil and the atmosphere;
the marine system which exchanges carbon within the oceans and between the oceans and the atmosphere.
Together they form the natural carbon cycle.
4.2 The terrestrial carbon cycle
Figure 1.10 shows the rates of natural carbon exchange between the terrestrial system and the atmosphere.
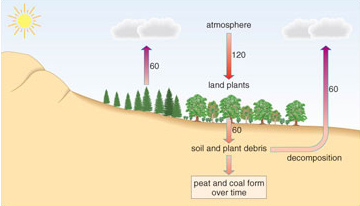
Question 9
Using the data in Figure 1.10, calculate whether there is a net movement of carbon into or out of the atmosphere, as far as the terrestrial carbon cycle is concerned.
Answer
Figure 1.10 shows that 120 × 1012 kg yr−1 flows from land plants and soil and plant detritus into the atmosphere, and 120 × 1012 kg yr−1 moves from the atmosphere into land plants. Therefore, as far as the terrestrial carbon cycle is concerned, there is on average a net balance of carbon flow. (We show later how special conditions allow accumulations of plant material.)
The average time that carbon stays in a reservoir before moving to another reservoir is known as the residence time, and is measured by the amount of carbon in the reservoir divided by the transfer rate of carbon between it and other reservoirs.
Question 10
What is the average residence time of land-derived carbon in the atmosphere, which contains about 760 × 1012 kg of carbon?
Answer
The residence time of terrestrial carbon in the atmosphere, as defined above, is 760 × 1012kg/120 × 1012 kg yr−1, or just over 6 years, on average.
As Figure 1.10 shows, each year land plants take 120 × 1012 kg of carbon from the atmosphere. In the next section we consider how exactly carbon is exchanged between the atmosphere and plants.
4.3 Photosynthesis, respiration and decay
Green plants absorb solar radiation and use its energy to fuel photosynthesis — a chemical reaction in which carbon dioxide (CO2) from the atmosphere is combined with water (H2 O) to form carbohydrates with the general formula CnH2nOn. One of the simplest carbohydrates, glucose, has the chemical formula C6H12O6, so in its simplest form photosynthesis can be represented by the balanced chemical equation:
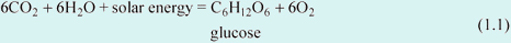
The oxygen produced by this reaction is released by plants into the atmosphere. Carbohydrates act as a store of energy for plants and also for other organisms that eat them. Such organisms use oxygen from the air to react with the carbohydrates (and other substances) to liberate energy by a process called respiration. During respiration, carbon dioxide and water are returned to the atmosphere. Expressed in the simplest chemical terms, the balanced reaction is:

Carbon exchanges or fluxes link the chemistry of the atmosphere with plant and animal chemistry. The carbon taken from the atmosphere (fixed) by plants enables them to grow, but in addition much of it enters the food chain as either living or dead material. Living plants are eaten by herbivores which themselves may become food for carnivores. The dead material provides food for the decomposers (bacteria and fungi) that live in plant detritus, in the soil, and on the rotting remains of dead animals. Almost all organisms return some carbon to the atmosphere through respiration, but by far the greatest contribution comes from the activities of the decomposers. The timescale by which this takes place is measured in months and years, so plant and animal material is not normally available to be preserved as fossil fuels.
However, if organic matter decays in an environment where the oxygen supply is limited, carbohydrates cannot be broken down completely to form water and carbon dioxide. In this special oxygen-poor (anoxic) environment, a carbohydrate comparatively enriched in carbon may be produced. For example, within the waterlogged environment of a swamp (mire), cellulose (a common constituent of plants) can be broken down according to the following reaction:
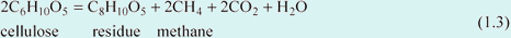
The residue produced, C8H10O5 is relatively enriched in carbon compared with the original cellulose (C6H10O5). This breakdown reaction releases methane (CH4), as well as carbon dioxide and water. Methane is an organic compound containing carbon and hydrogen but no oxygen; one of a family of organic compounds known as hydrocarbons. So anoxic environments prevent some fixed carbon returning to the atmosphere as CO2, and these hydrocarbons together with carbon-rich residues represent a chemical half-way-house within the carbon cycle, which make carbon available to form the basis for fossil fuels.
Although significant layers of decaying plant debris are found on the floor of modern forests, these are often oxygen-rich environments thanks to the constant reworking of decaying material by plants and animals, fungi and bacteria. However, one modern environment with which you are probably familiar does contain plant material decaying in anoxic conditions — the peat bog.This is where useful preservation of terrestrial carbon occurs.
4.4 The marine carbon cycle
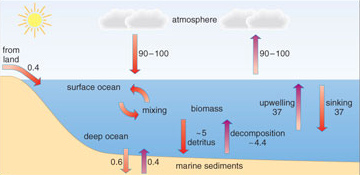
The ocean stores much more carbon than the terrestrial system (Figure 1.9). How is this marine carbon fixed into organic carbon within the sediments, and what are the main reasons for marine carbon fluxes? Figure 1.11 shows the rates of natural carbon exchange within the ocean and between the ocean and the atmosphere.
Question 11
From the data presented in Figure 1.11, is there a net movement of carbon into or out of the atmosphere from the ocean?
Answer
Figure 1.11 shows that 90-100 × 1012 kg of carbon flows each year from ocean surface waters into the atmosphere, while roughly the same amount of carbon moves from the atmosphere into the ocean: there is no net movement.
Carbon dioxide is constantly being exchanged between the atmosphere and the upper levels of the oceans, by physical and by chemical processes. At the base of the food chain that produces organic material in the oceans are the marine phytoplankton (microscopic water-borne plant life) which require dissolved CO2 for photosynthesis. The process also requires solar energy, so phytoplankton can live only in the sunlit upper parts of the ocean. Their photosynthesis releases oxygen, which dissolves in seawater. The productivity of phytoplankton depends on sunlight, temperature and supply of nutrients, and therefore varies geographically.
Carbon dioxide is more soluble in cold water than it is in warm water, so the concentration of dissolved CO2 tends to be higher in cold polar waters than in warm tropical waters. Cold, dense polar water sinks and flows under the influence of gravity along the ocean floor towards the Equator. It returns to the surface by upwellings at various places in the oceans, to supply nutrients and promote unusually high phytoplankton productivity there.
Zooplankton (water-borne animal life, mostly microscopic) and higher marine organisms consume these phytoplankton. The dead remains of phytoplankton, zooplankton and larger organisms sink through the water column, transferring carbon from the upper few hundred metres towards the ocean depths. However, little of this organic matter gets a chance to accumulate on the ocean floor. It provides food for filter feeders in deep water and on the ocean floor, and through them for predatory animals, and ultimately feeds the ubiquitous decomposers. All these organisms release CO2 back into solution through respiration. As Figure 1.11 shows, only a small proportion of the marine carbon cycle, 0.2 × 1012 kg yr−1 of carbon, is incorporated into marine sediments.
Question 12
Assume that 0.2 × 1012 kg yr−1 of carbon were incorporated into marine carbonates and fossil fuels in proportion to their present-day amounts (Figure 1.9). How long would it have taken to deposit all the carbon found in the current global store of POC, of which fossil fuels are a part?
Answer
We know from Figure 1.9 that the global carbonate store is some 4 × 1019 kg of carbon, and that the POC store is some 1019 kg of carbon, a total of 5 × 1019 kg of carbon. If carbon were deposited into each reservoir in proportion, the rate of deposition of carbon into POC would be:

At this rate, it would have taken 1 × 1019 kg/0.04 × 1012 kg yr−1, which is 2.5 × 108 years, i.e. 250 million years to deposit enough carbon to form the global POC store, which includes fossil fuels.
However, not all fossil fuel formed in the marine carbon cycle; much of it formed on land (Section 4.3). The preservation of organic material in sediments depends not only on the supply of dead organisms, but also on anoxic chemical conditions where they accumulate.
4.5 Generating carbon — the legacy of volcanoes
What is the origin of the carbon within the carbon cycle? Figure 1.9 showed that the greatest proportion of the global carbon store is locked into carbonate rocks. Over the 4.5 billion years of the Earth's history, carbon must have moved from the atmosphere into the oceans and thence into carbonates. How did the atmospheric carbon originate?
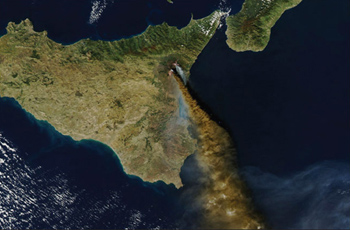
The Earth's atmosphere as a whole was derived mainly from gases brought to the surface from the Earth's interior. For example, the 1991 eruption of Mount
Etna in Sicily released an estimated 0.01 × 1012 kg of carbon in the form of CO2 (Figure 1.12). Most volcanic carbon comes from the steady degassing of lava flows rather than from volcanic vents.
In the short term, volcanic sources release insignificant volumes of CO2 compared with other fluxes of carbon, but over geological time, degassing of the Earth's interior can reasonably account for all the carbon in the natural surface system.
4.6 The fossil fuel 'bank'
During the period of accumulation of most coal and petroleum, the past few hundred million years, the equivalent of around 1023 J of chemical energy have been 'banked' by Earth processes. As you have seen in Sections 4.3 and 4.4 carbon is added to these reservoirs continually, at a rate today that is equivalent to about 5 × 1017 J yr−1. That rate of growth represents roughly a mere thousandth of present world energy demand.
In fact, the 'bank' contains only about 0.04% of the amount of organic carbon preserved in sedimentary rocks. However, that total store of buried organic carbon is finely dispersed, at an average concentration of about 0.4% in sedimentary rocks. At that level, it can never be exploited, either economically or with an efficiency that yields more energy than it consumes.
Although buried organic carbon is widely distributed, concentrations sufficient to constitute resources are rare and very restricted in space and geological time relative to small amounts of organic carbon that are present in many sediments.
5 Nuclear energy
Einstein's famous equation E = mc2 shows that mass (m) and energy (E) are proportional to one another. The constant c2 linking the two is the square of the speed of light c (3 × 108 m s−1). Implicit in the equation is that mass can be converted into energy, and vice versa, although the conversion of energy into mass occurs only in very powerful particle accelerators. The conversion of matter into energy is the basis of nuclear energy. When unstable nuclei split (nuclear fission) the sum of the masses of the isotopes that are produced is slightly less than that of the original fissile isotope. That tiny mass deficit is converted into a relatively huge amount of energy, because c2 is a very large number (9 × 1016 m2 s−2). When isotopes fuse at immense temperatures in the interiors of stars, the product again has lower mass than the original isotopes. So both nuclear fission and fusion potentially produce energy that can be exploited, but in both cases the phenomena have to be artificially induced.
The 'fuel' for nuclear fission occurs naturally in the Earth's crust in the form of unstable isotopes of uranium and thorium, added to by other isotopes that are formed artificially inside nuclear reactors. Note that uranium and thorium isotopes naturally break down by emission of various particles (helium nuclei and electrons), as a result of which their 'daughter' isotopes have lower atomic masses and numbers. Some of the 'daughter' isotopes are also unstable and undergo radioactive decay, which eventually results in the formation of several stable isotopes of the element lead. Such radioactive decay is not the same as nuclear fission and occurs at a constant pace. Radioactive decay also releases energy, but at an amount far lower per decayed atom than in nuclear fission. That energy continually heats the Earth's interior (Section 3.3) and is the source of geothermal energy.
Potential 'fuel' for nuclear fusion also occurs naturally in the form of an isotope of hydrogen that includes a neutron as well as a proton in its nucleus, instead of the single proton. This isotope (2H or deuterium) occurs in tiny amounts in water, and was produced originally by processes early in the history of the Universe.
6 Concentrating, storing and transporting energy
The Earth is awash with energy from sources other than fossil fuels; thousands of times as much as humans use. Why then do we need to use any other energy supply?
6.1 Concentrating energy
As far as human needs are concerned, there is a marked difference between 'dilute' and 'concentrated' energy. Water vapour in the atmosphere, for example, has considerable potential energy since a huge mass globally (about 13 × 1015 kg—Smith, 2005) is held high above the Earth's surface. But this potential energy represents a very dilute form of energy; falling rain could not turn a water wheel. It is only when energy can be 'concentrated' that it can be put to good use — in this case by rainfall accumulating in streams and rivers, or being stored in reservoirs at high elevations. The concentration can be expressed colloquially in terms of energy density, which is the amount of energy stored by a resource divided by the volume of the space that it occupies.
Some forms of energy are relatively difficult to concentrate, so have a low energy density, whereas others are easier to concentrate. The energy contained in moving air is rather difficult to concentrate; windmills and wind farms have to be sited where natural factors enhance wind speed and constancy. Solar power has a low energy density, so requires large collecting devices. The potential energy of rain is naturally concentrated and held in mountain lakes; we concentrate this energy artificially when rainwater is stored in a reservoir. This emphasises why fossil fuels are so valuable as they represent naturally concentrated forms of the solar energy that reached the Earth millions of years ago.
The ultimate form of concentrated energy is matter itself, in the form of nuclear energy.
6.2 Storing and transporting energy
To be useful to us, energy must be available where and when we want it, and in a form and in amounts we can handle. Severe weather systems concentrate natural energy wonderfully, but hurricanes associated with storm-force winds, driving heavy rain, thunder and lightning wreak havoc rather than top up our energy supplies.
Storing most forms of energy is very difficult. We have to re-heat our homes daily in the wintertime because they constantly lose heat, despite our attempts to insulate them. We cannot store light when the Sun goes down; we have to turn electricity into light until the Sun reappears. In fact, only two forms of energy are truly storable. Potential energy can be stored almost indefinitely by mechanical means, as in springs or lifted weights — the basis of clocks. Far more convenient is storage that exploits chemical energy — batteries, or even better, chemical fuel.
Fuels are compounds whose combustion liberates a large amount of energy per unit mass: they commonly have a high energy density. Wood was the major fuel before the Industrial Revolution, and remains the most important fuel for many non-industrial societies today. Wood, and other plants that can be used as fuels, produce biomass energy. As industry develops, energy demands grow and fuels with higher convertible energy content per unit mass are needed. Modern energy supply is centred on the fossil fuels: coal, oil and gas (Figure 1.4). Note that although the isotopes whose fission or fusion forms the basis for nuclear power are not burnt, they are generally known as nuclear fuels.
A further advantage of fuels as energy sources is their transportability, so that conversion can take place on selected sites or in mobile units. Highly concentrated fuels require less energy to transport than those with a low energy density but since lots of energy can be released accidentally from badly handled concentrated energy sources, care has to be taken to ensure that transport is safe. For some applications, such as cars, generating energy from stored chemical energy has the advantage of the ease of transport of small amounts of fuel.
Fossil fuels therefore represent extremely useful energy sources because they have a high energy density, they store energy for very long periods to be used when needed, and they can be transported simply and relatively safely.
A very important form of energy transport in developed countries is the generation and transmission of electricity. Figure 1.6 shows that around one-sixth of energy used in the UK is distributed as electricity via the National Grid. Since every primary energy source is used to some extent in generating electricity, a brief introduction to generators is useful (Box 1.2).
Box 1.2 Electricity generation
By far the greatest contribution to electricity supplies is made by exploiting the way in which an electrical current is induced in a conductor when it is moved through a magnetic field. Most such generators are made up of a cylindrical coil of conducting wire that is rotated between extremely strong magnets, so that an electrical current is induced in the coil (Figure 1.13a). There are other means, such as fuel cells and photovoltaic devices, but generators are common to conversion of most kinds of primary energy. Driving the rotation depends on harnessing energy released from primary energy sources through a specific form of engine, the turbine.
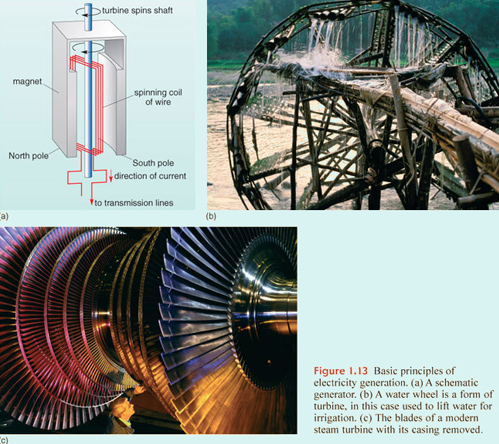
A turbine is a rotary engine driven by a moving fluid. A propeller on a boat or an aircraft, or a jet turbine, provides a driving force because of the design of its blades. A turbine used in electricity generation simply exploits the reverse of this effect. Moving 'fluid' provides the force that spins the turbine. The rotation transmits energy, and for electricity generation it is connected to a rotary generator. The simplest turbines have one moving part, as in windmills or water wheels (Figure 1.13b).
Electricity generation from flowing water or wind exploits the kinetic energy of the fluid. However, from the 19th century to the present day, the most common turbines used to generate electricity have been driven by high-pressure steam created by boiling water using coal, oil, natural gas, nuclear or geothermal energy (Section 5.1). In some electricity generating stations the moving fluid is the gas produced by burning oil or natural gas, in the manner of a jet turbine, thereby missing out the intermediary of steam and the associated inefficiency. The principle of a steam turbine is somewhat different from those used to harness the energy of wind and flowing water, and so too is its design (Figure 1.13c). Steam contains energy in three forms: as heat; as the energy needed to change water's state from liquid to gas ( latent heat of vaporisation); and as the energy bound up by the compression of a pressurised gas. The last is a form of potential energy, released when the pressure drops, as in the case of the air in a tyre when its valve is opened. Together, these three forms of contained energy are known as the enthalpy. The enthalpy of a gas is given by:
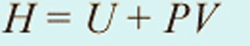
where H is enthalpy (in J), U is heat (including latent heat of vaporisation; in J), P is pressure (in pascals or kg m−1 s−2) and V is the volume of steam (in m3). (Note that pressure times volume — in kg m2 s−2—gives units in joules.) It is the PV term that provides much of the driving force for a steam turbine, because the pressure gradient across the turbine leads to rapid expansion of the steam and therefore high speed in the flow. Many steam engines, including the original piston engines invented in the early Industrial Revolution, also exploit the latent heat of vaporisation, by condensing the steam at the outlet of the turbine. The change of state from gas to liquid results in a near vacuum that increases the pressure gradient across the turbine — condensation adds 'suction' to the 'blowing' by steam. Latent heat is released and this can be 'recycled' in the generating system.
The late-20th century saw an increased reliance on electricity generation that uses natural gas as a fuel. Gas turbines are similar in design to those used in aircraft jet turbines. Turbines rotated by combustion gases have two important advantages over steam turbines: they are more efficient, and can be turned on and off very quickly, thereby suiting variable demand for electricity. Natural gas also contains a lower proportion of carbon than coal and oil, and so less carbon dioxide is emitted to the atmosphere.
7 Renewable and non-renewable energy supplies
Energy resources can be considered in a completely different way from their energy density — whether or not they are renewable. Some energy sources incorporate energy released comparatively recently from the Sun and are replenished naturally over a timescale of days to tens of years. Therefore solar, wind and wave energy resources, being continually available, are renewable energy supplies. Other examples of renewables are geothermal and tidal energy sources.
Other potential energy resources are legacies of solar power received and converted into stored energy in the geological past; coal is a good example. Coal seams are replenished naturally over a timescale of millions of years. Once coal began to be exploited faster than its rate of creation it became non-renewable and its use at current levels cannot be sustained.
Question 14
Which energy sources mentioned so far are renewable, and which are nonrenewable on the scale of a human lifetime?
Answer
Energy sources such as solar, biofuels, hydro, wind, waves, tides, and geothermal are continually replenished, so they are renewable on this timescale. The fossil fuels and nuclear energy are not being replaced on this timescale, so they are non-renewable.
All fossil fuels are slowly being renewed by the death, burial and decay of present plant and animal life, but at an extremely slow rate compared with the pace of human economic activity. The Earth's natural systems may eventually replace all the fossil fuels that humanity has already used, but how and when that might become possible is impossible to judge.
Likewise, only extremely slow geological processes renew fissionable nuclear fuels. The instability of naturally radioactive isotopes such as uranium and thorium, which formed in stars much larger than the Sun during the moment of their destruction as supernovae, results in them gradually diminishing with time. Replenishing the ores of uranium and thorium by geological processes takes hundreds of million years. So, nuclear fuels too are non-renewable. Even the hydrogen isotope deuterium, which is potentially a vast source of energy from thermonuclear fusion, is a 'one-off' legacy resource and is finite. But even so its potential vastly outstrips that of all other non-renewable energy sources.
The distinction between renewables and non-renewables is one of timescale and energy concentration, but it is critical for human society. Think of renewable energy resources as income, and non-renewable energy resources as inheritance. We 'spend' the Earth's energy resources constantly for cooking, travelling, heating or cooling buildings, manufacturing and in many other ways. At present, modern industrial societies generate energy mostly from fossil fuels, thereby depleting an inheritance accumulated from millions of years of 'banked' solar energy and internal heat. Much less energy is currently generated from day-to-day energy 'income', i.e. from renewables: the global energy 'accountancy' has become unbalanced and unsustainable.
Setting aside the environmentally damaging effects of burning fossil fuels, such as atmospheric pollution and global warming, sooner or later present energy generating policy will deplete our stock of fossil fuel. To stay solvent in energy terms over the long term leaves no choice other than to transfer society's day-to-day energy supply to renewable sources, or return to a low-energy society. The other alternative is harnessing energy from nuclear fission or fusion, but that too cannot last indefinitely. This book aims to provide a scientific basis to understanding some of the decisions that will need to be made to enable humanity to stay 'solvent' in energy terms. Yet you will no doubt be well aware that such decisions are not those of individuals, but are presently dominated by economic and political factors, irrespective of the scientific facts.
Conclusion
Energy is the basis of modern society. Other physical resources can only be effectively extracted, processed and transported if there is a ready supply of energy at the right price.
Energy is defined as the capacity to do work, while work is a force acting on an object that causes its displacement (i.e. force × distance). Both energy and work are measured in joules or, more fundamentally, in newton metres. Power (measured in watts, i.e. joules per second) is the rate of doing work or the rate at which energy changes from one form to another.
All conversions of energy are inefficient to varying degrees, so that primary energy consumption far exceeds the useful work that it makes possible.
The Sun is by far the most important source of natural energy on Earth. The solar radiation that reaches the Earth contributes to winds, waves, atmospheric water circulation, atmospheric heating and surface water evaporation, and to organic activity.
The gravitational attractions of the Sun and the Moon combine to produce tides, and rocks in the Earth's interior also generate heat by the decay of radioactive isotopes in them. These are small but potentially exploitable sources of energy.
Fossil fuels are ultimately derived from solar radiation, through photosynthesis and the carbon cycle.
Most of the world's carbon is locked within carbonate rocks. A large amount of carbon also exists as preserved organic carbon, which includes fossil fuels.
Green plants use solar radiation to build carbohydrates and plant tissue from carbon dioxide and water in the atmosphere and dissolved in the oceans, in a process known as photosynthesis. Photosynthesis releases oxygen into the atmosphere and oceans. When they respire, organisms use oxygen to generate energy from food, releasing carbon dioxide and water vapour back into the atmosphere and oceans. These respiratory reactions are the reverse of photosynthesis.
Concentrations of marine phytoplankton occur in the upper sunlit layers of the oceans where upwelling currents bring nutrients. These form the basis of the marine food chain. There is only a build-up of carbon within marine sediments where there is an adequate supply of organic material and where physical conditions are right for its preservation.
Nuclear energy is derived from the conversion of matter into energy and has a very high energy density.
The world is plentifully supplied with solar-derived energy, but most of it is not in a sufficiently concentrated form to be useful to modern industrial society.
Fuels are of immense value because they are concentrated sources of energy (they have a high energy density) that can easily be stored, transported and used at will. At the current level of technology, energy transport has become an essential aspect of energy use, dominated by electricity. The most convenient way of electricity generation is through the conversion of mechanical motion — most usually produced today from thermal energy — using generators driven by turbines.
Energy sources can be subdivided into renewables, like solar, wind and wave power, and non-renewables, like peat, coal, oil and gas. Renewables are effectively everlasting, but non-renewables are finite.
Acknowledgements
Course image: Adrian S Jones in Flickr made available under Creative Commons Attribution-NonCommercial-ShareAlike 2.0 Licence.
Grateful acknowledgement is made to the following sources for permission to reproduce material in this course:
The content acknowledged below is Proprietary (see terms and conditions) and is used under licence (not subject to Creative Commons licence ).
Figure 1.2 National Railway Museum/Science and Society Picture Library
Figure 1.5a BP (2004) Statistical Review of World Energy 2004
Figure 1.5b Adapted from NASA material
Figure 1.8 Martin Bond/Science Photo Library
Figure 1.12 NASA's Earth Observatory
Figure 1.13b © David Sanger Photography/Alamy
Figure 1.13c Steve Allen/Science Photo Library
The information in this book has been obtained from a wide range of sources, too numerous to mention. However, specific reference is made in the text to the following:
Boyle, G., Everett, B. and Ramage, J. (eds) (2003) Energy Systems and Sustainability, Oxford University Press and The Open University.
Statistics on UK transport. Available online from Department for Transport Statistics [last accessed December 2007].
Harris, S., Bridgeman, F. and O'Reilly, T. (eds) (2002) Britain's Offshore Oil and Gas, UK Offshore Operators Association. Available online at Oil & Gas UK [last accessed April 2011].
Smith, S. (2005) Water: The Vital Resource (Book 3 of S278 Earth's Physical Resources: Origin, Use and Environmental Impact), The Open University, Milton Keynes.
© Skyscan/Science Photo Library
All other material contained within this course originated at the Open University.
This resource was created by the Open University and released in OpenLearn as part of the 'C-change in GEES' project exploring the open licensing of climate change and sustainability resources in the Geography, Earth and Environmental Sciences. The C-change in GEES project was funded by HEFCE as part of the JISC/HE Academy UKOER programme and coordinated by the GEES Subject Centre.
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
Copyright © 2016 The Open University