Energy resources: Solar energy
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Thursday, 25 April 2024, 4:17 PM
Energy resources: Solar energy
Introduction
Energy from sources other than fossil or nuclear fuels is to a large extent free of the concerns about environmental effects and renewability that characterise those two sources. Each alternative source supplies energy continually, whether or not we use it. Many alternative sources of energy have been used in simple ways for millennia, e.g. wind and water mills, sails, wood burning - but only in the last two centuries has their potential begun to be exploited on an industrial scale. Except for geothermal energy, all have their origins in energy generated outside the Earth, yet the potential of each is limited by its total supply set against its rate of use. Each is likely to be renewable in the sense that the available rates of supply of each exceed those at which they are used. The main concern is whether or not such alternatives can supplant fossil- and nuclear-fuel use to power social needs fast enough to avoid the likelihood of future global warming and other kinds of pollution.
One of the alternative sources to consider is solar energy.
The Sun will radiate energy until it ceases thermonuclear fusion, in around 5 billion years. About 33% of the solar power that enters the Earth's system heats the atmosphere and contributes to setting winds and waves in motion. Of that reaching the Earth's surface, 70% falls on the sea, setting in motion ocean currents and a large proportion of the circulation of water vapour in the atmosphere because of evaporation from the ocean surface. The remainder falls on the land. Solar energy is redistributed through interlinked surface systems:
- the carbon cycle based on photosynthesis;
- atmospheric circulation and the water cycle;
- winds and ocean waves; and the ocean current system.
Each of them is a potential source of useable energy. In every case, with the exception of the energy available from surface water flow, humanity comes nowhere near exploiting the Sun's potential to supply useable energy; in fact, we really do not know the practical limits. Whatever those are, they will not disappear as a resource - all are renewable. Compare this with the solar energy stored chemically by the degraded products of photosynthesis in fossil fuels. Although carbon burial adds continually to that resource, its pace of renewal (between 1 to 10 GW - Figure 1) is about 2000 times slower than we use it. Fossil fuels are non-renewable and declining extremely quickly in terms of human history.
This course explores Solar power as a source of directly useable energy.
This OpenLearn course provides a sample of level 2 study in Science.
You may also be interested in our badged course Can renewable energy sources power the world?.
Learning outcomes
After studying this course, you should be able to:
explain the principles that underlie the ability of various natural phenomena to deliver solar energy
outline the technologies that are used to harness the power of solar energy
discuss the positive and negative aspects of solar energy in relation to natural and human aspects of the environment.
1 Solar energy
The Sun will radiate energy until it ceases thermonuclear fusion, in around 5 billion years. The solar power that enters the Earth's system is 1.1 × 105TW (0.3 × 105 TW to atmospheric heating and 0.8 × 105 TW absorbed at the surface - Figure 1). This is equivalent to a global energy supply of 3.5 × 106 EJ yr−1. About 33% heats the atmosphere and contributes to setting winds and waves in motion. Of that reaching the Earth's surface, 70% falls on the sea, setting in motion ocean currents and a large proportion of the circulation of water vapour in the atmosphere because of evaporation from the ocean surface. The remainder falls on the land. Figure 1 shows the complexity of primary redistribution of solar energy through interlinked surface systems:
the carbon cycle based on photosynthesis;
atmospheric circulation and the water cycle;
winds and ocean waves; and the ocean current system.
Each of them is a potential source of useable energy: this course discusses the direct use of solar energy.
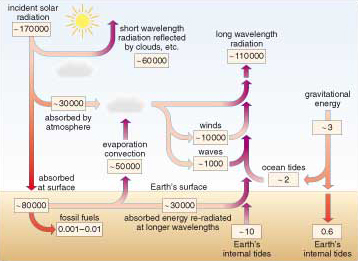
In every case, with the exception of the energy available from surface water flow, humanity comes nowhere near exploiting the Sun's potential to supply useable energy; in fact, we really do not know the practical limits. Whatever those are, they will not disappear as a resource - all are renewable. Compare this with the solar energy stored chemically by the degraded products of photosynthesis in fossil fuels. Although carbon burial adds continually to that resource, its pace of renewal (between 1 to 10 GW - Figure 1) is about 2000 times slower than we use it. Fossil fuels are non-renewable and declining extremely quickly in terms of human history.
In British summers direct sunlight (insolation) on a clear day can reach power densities of up to 1 kW m−2 when the Sun is at its highest in the sky (the daytime average is about 450 W m−2), and supplies around 6.5 kWh m−2 per day (450 W m−2 × about 14 hours of daylight in June) (Figure 2a). This is enough to heat a bathful of water to a comfortable temperature. Summer insolation in southern Europe (~30° N on Figure 2a) reaches 7.5 kWh m−2 per day (by virtue of a higher Sun angle). Winter insolation is much lower. In January, northern Europe (~60° N on Figure 2a) has an average insolation around 10% of its summer value and around only 20% of the January value in southern Europe. In the tropics (0° N on Figure 2a), insolation shows far less seasonal fluctuation. Solar energy collection in northern Europe is therefore most effective in the summer, in southern Europe there is useful winter potential as well, and at low latitudes it can be used throughout the year. High latitudes have a problem with solar energy; it is most abundant when it is least needed, i.e. in summer. Although variations in cloudiness reduce insolation, diffuse solar energy still reaches the surface at a power density that is often useable.
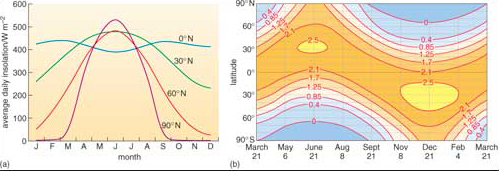
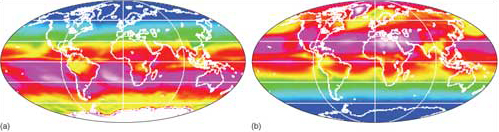
Curvature of the Earth's surface and the inclination of its rotational axis relative to the Sun result in insolation declining from a maximum in the tropics towards higher latitudes (Figure 2b). There is also considerable variation with the seasons. Satellite data give a more realistic view of the amount of available solar energy across the planet (Figure 3). One surprising feature is that summer months in Greenland and Antarctica have the highest actual insolation on the planet. That is partly because the Sun shines for 24 hours each summer day, but also because ice caps are usually free of cloud during summer months - high-pressure air masses exclude moist air from the oceans. (Note: Much is reflected away by ice and snow.) Bar that oddity, available solar energy varies roughly as expected, but always lower than theory predicts (Figure 2) because of cloud cover.
Direct solar energy use is of two basic kinds; using it as a source of heat or converting it into electricity using photovoltaic technology.
2 Solar thermal energy
Solar heating of trapped air, water and solids has been used for centuries, but modern architectural design can enhance all three effects for space heating, hot water supply and heat storage. Such passive solar heating relies on short-wave radiation being absorbed by materials so that they heat up and then slowly re-emit long-wave radiation. The most obvious example is inside a greenhouse, where solar radiation that passes through the glass heats the inside air to temperatures well above those outside. Glass is more transparent to short-wave radiation than to long wavelengths, so a glazed room absorbs and retains heat, especially if the windows face the Sun. By absorbing solar radiation, house bricks, beach sand and rocks can become uncomfortably hot, even in the UK. The darker solids are, the more short-wave radiation they absorb - black cars become hotter inside than do white ones on sunny days.
The most common design using water as a heat absorber is a flat glass plate set above a waterproof black surface (Figure 4a). Water flows slowly between the two, becomes heated and can either be stored in tanks or pumped through radiators for space heating. Greater efficiency is achieved by the use of fluids that are more volatile than water. They are vaporized in the gap and then pass through a condenser, where the gas to liquid transformation releases the latent heat of vaporization imparted to the gas by solar energy; a form of heat pump. Some solids require more energy to raise their temperature than others; they have a high specific heat capacity. If this property is combined with that of slow release of energy - because of high density and low thermal conductivity -such solids are ideal for night-storage heaters. Conventionally, such storage devices are heated by electricity, but can be used in walls to absorb solar energy. The more massive they are, the more energy they absorb, and the longer they are capable of heating spaces. Used in well-insulated buildings, solar energy can be the sole means of space- and water-heating.

Passive solar heating systems first appeared during the 19th century, particularly in the southern states of America, where water filled tanks were warmed behind glazed walls. As cheap and more convenient oil and gas came to dominate domestic heating during the 20th century this bulky approach all but disappeared. Since the late 1960s, growing environmental consciousness has brought renewed interest in solar heating. By 2001, 57 million square metres of simple solar collecting panels (Figure 4a) had been installed globally, mainly in industrialized countries. That they are not even more widely used reflects their high price, driven by high demand relative to a restricted number of suppliers. Despite current high costs of installation, in the long-term almost totally free energy input makes passive solar heating a good investment.
A more technically advanced exploitation of insolation uses mirrors to focus solar radiation. Active solar heating is increasingly used in poor countries for cooking (Figure 4b), thereby reducing the demand for fuelwoods and the risk of cancers from smoke inhalation. Similar, roof-mounted devices heat water for domestic use or radiators to much higher temperatures than does passive solar heating. On a larger scale, arrays of large reflectors boil water to power turbines and generate electricity, or create extremely high temperatures in solar furnaces (Figure 4c). The mirrors are motorized and controlled so that they track the movement of the Sun.
3 Photovoltaic conversion of solar energy
You have probably used direct conversion of solar energy to electricity though a solar cell that powers a pocket calculator or a solar 'trickle' charger to top up car batteries. Both exploit the photovoltaic (PV) effect, first described in 1839 by Edmond Becquerel. He observed that the voltage of an early 'wet cell' battery increased when its silver plates were exposed to light. In 1877 Cambridge physicists discovered that selenium crystals created an electrical current when exposed to light. A New York electrician, Charles Edgar Fritts, devised the first working PV cells in 1883, using selenium plates covered in gold wires, but they converted less than 1% of the incident solar energy into electricity. Since then PV technology has been mainly concerned with improving efficiency, so that today's solar cells convert up to 25% of incident solar power to electricity.
In the 1950s the Bell Telephone Company's laboratory in New Jersey, USA experimented on the effect of light on semiconductors, that had been invented as miniature analogues of the valves used in radios. These first transistors were made of silicon that had been 'doped' with minute amounts of other elements, including boron or phosphorus. The impurities increase the electrical conductivity of poorly conductive silicon to that intermediate between non-conductors and metals -hence the name, semiconductor.
The outer electron shell of a silicon atom is only half filled, leaving four unfilled electron sites. In crystalline silicon the atoms share their electrons. Consequently, pure crystalline silicon is a poor conductor of electricity because the outer electrons are barely able to move.'Doping' the silicon with elements whose atoms have different numbers of outer electrons modifies this reluctance to conduct electricity.
Phosphorus, with five electrons in its outer shell, is one example of a doping element. For each phosphorus atom that bonds with an adjacent silicon atom there is an excess electron. So silicon doped with phosphorus has a slight overall negative charge; it is an n-type semiconductor. When a photon of light meets a doped silicon-phosphorus pair the weakly bonded,'excess' phosphorus electron breaks free and moves. Passage of electrons through the silicon lattice creates a weak electrical current and an electrical potential.
Silicon doped with boron is left with unfilled electron shells, since boron has three outer electrons, leaving the boron-doped silicon with a slight positive charge; this is a p-type semiconductor that is able to accept electrons. Layering together both n- and p-type semiconductors in wafers (Figure 5a) enhances the PV effect. Negatively charged electrons from the illuminated n-type cross the gap between the wafers to restore electrical equilibrium. However, despite the high electrical potential across the gap, large positively charged silicon atoms cannot cross it, and so only electrons can flow. The resulting current is conducted to its point of use through metal ribbons embedded in the PV wafer, which inevitably block some incoming photons. Silicon is highly reflective, so an antireflection coating is applied to the cell surface to maximize photon capture. Doped wafers and electrical conductors are generally mounted in a vacuum between glass sheets as solar PV cells for assembly in tiled arrays (e.g. Figure 5b).
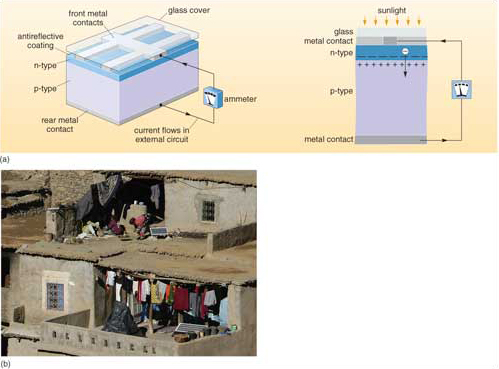
Solar PV cells based on doped crystalline silicon have a theoretical maximum efficiency of 28%, because only a narrow range of short wavelength radiation from the Sun has the appropriate energy to displace electrons. In practice, they convert about 15% of the incoming solar energy in that wavelength range to electrical current. Crystalline silicon is durable but expensive to manufacture. Fine-grained, doped silicon formed into wafers is cheaper, but its efficiency is around 5 to 7%, and it eventually degrades. Current research centres on low-cost semiconducting materials, one promising candidate being fine-grained titanium dioxide - the white pigment used in paints, and therefore cheaply available. Combined with dyes to increase the sensitivity of TiO2 to sunlight, it has a practical PV efficiency of 7 to 12%, and degrades very slowly. Titanium dioxide occurs as a natural mineral (rutile - TiO2), concentrated in some beach sands because of its high density. Titanium dioxide embedded in thin plastic films may prove to be a much cheaper alternative to silicon cells. Research into PV materials is understandably a 'hot topic', and among novel possibilities are organic compounds that behave as PV semiconductors, and could be formed into flexible and strong solar cells.
What is the main practical problem that might inhibit the widespread deployment of solar cells? (Think of your household electricity supply.)
Direct current (DC) is produced, whereas all appliances linked to the 'mains' use alternating current (AC) generated by conventional turbines. Photovoltaic cells need DC-AC inverters to supply alternating current to the grid system (see Chapter 1.
Another drawback of photovoltaic solar power is the area needed to give a useful supply; a 1 m2 solar panel operates at an average power of about 30 W, so three are needed to light one 100 W light bulb. To put this in perspective, to supply the entire electricity needs of New York City with solar power would require solar cells covering an area of 192 km2; equivalent to about 25% of the city's area.
4 The future of direct solar energy use
The immense global energy flux from the Sun makes it the prime candidate for future sustainable energy production. Both solar thermal energy and solar PV conversion involve technologies that can be deployed on personal through community to regional scales, using both simple and advanced technologies. You have probably seen solar PV panels that power automatic roadside weather stations and other low-drain communications systems. The panels require low maintenance and usually charge batteries to allow them to remain operational during the night. In poor countries where the energy infrastructure is rudimentary or absent, PV systems hold out great potential. An important use is for daytime pumping of water from wells.
In its 1997 renewable energy plan, the EU set a target of half a million villagescale direct solar systems to be deployed in developing countries and a similar number in European houses by 2010. The United Nations has asked world governments to deploy 4.5 GW of solar PV electricity generating capacity in developing countries by 2012. Both Japanese and German governmental subsidies have boosted both photovoltaic production and deployment, and a similar 'hard-sell' stems from commercial sources in the US. As a result, power capacity of solar PV rose from a global 50 MW in 1995 to over 2 GW in 2002, and is estimated to be growing at a rate of 40% per year. The main hindrance to greater deployment is simply that of cost; at between US$ 0.2 to 0.5 per kWh, solar PV electricity was almost ten times as expensive in 2005 as that from the cheapest fossil-fuel source, natural gas. To progress, the technology requires continued reduction in the cost of the solar cells themselves - but the enormous reduction in cost of silicon-based computer hardware since the 1970s is cause for optimism.
Solar PV could theoretically supplement grid-power during daylight hours to reduce generating costs and environmental emissions. However, at this scale serious disadvantages emerge. The daily intensity of sunlight varies dramatically because of cloud cover. Moreover, solar power is greater in the summer while the demand for electricity is lower, except in areas with high use of air conditioners. Provided photovoltaic conversion contributes no more than 10-20% of the total amount of electricity in the grid, its integration seems feasible. This is because electricity grid systems are designed to cope with large variations in demand, and they can cope equally well with fluctuations from different forms of supply.
Should future solar PV power rise above 20% of the total electricity supply, then existing grid systems built to be dominated by coal, oil and nuclear generation would have to be modified. This is because conventional power plants are slow to start up and shut down; they are slow-response systems. Solar conversion, along with other alternative sources whose power source fluctuates uncontrollably (e.g. wind and waves), is a fast-response system. A distribution grid with solar PV power as a major component would need to be supplemented by controllable fast response systems, principally hydroelectric and gas-turbine generators. Another solution would be short-term electrical storage installations, but they are both costly and inefficient. A means of 'having one's green cake and eating it', however, would be to use electricity from solar PV to generate hydrogen by electrolysis of water. Hydrogen gas is combustible, storable and moveable, and so avoids most of the problems associated with electrical storage. The hydrogen could even be converted back into electricity using fuel cells.
Despite these caveats, the potential of solar PV is enormous. If photovoltaic conversion with 10% efficiency was installed over an area of 500 000 km2 (about 1.3% of the area of tropical deserts) humanity's present energy requirements would be met. That outlook is probably far off. Of the electricity generated from all alternative energy sources in the early 21st century, solar PV contributes only about 0.02%, with solar thermal generation a little more significant at 0.06%.
5 Biomass conversion of solar energy
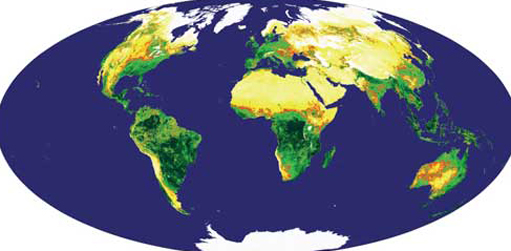
Photosynthesis in the geological past was responsible for all fossil fuel reserves, but its products are buried about 2000 times more slowly than we use them at present. The total carbon content of all biomass growing on land is estimated to be 5.6 × 1014 kg and, as Figure 10 shows, about one-fifth of this mass is renewed each year. Figure 6 shows how modern plant biomass is distributed across the continents. Clearly, biological conversion of solar energy to a chemical form in combustible materials presents a potentially vast resource. Using biomass as a fuel need not add to carbon dioxide in the atmosphere, because its products, CO2 and water vapour, are exactly the same as those of natural aerobic decay. If plant photosynthesis is carefully managed to renew carbon consumed by using vegetable matter as an energy source, the natural balance of the carbon cycle can be sustained. About 6% of the total biomass that grows annually - containing about 7.5 × 1012 kg of carbon content - would meet all humanity's current demands for energy. However, such a perspective has nightmarish overtones because its production would involve about 11% of the global land area (a much greater proportion of fertile areas - see Figure 6). Nonetheless, 'high-tech' harvesting of solar energy via photosynthesis is a potential alternative to fossil-fuel use. There are five main approaches to extracting biomass energy: solid fuel combustion; gasification; pyrolysis; digestion and fermentation.
Conclusion
Solar power is an immense source of directly useable energy and ultimately creates other energy resources: biomass, wind, hydropower and wave energy.
Most of the Earth's surface receives sufficient solar energy to permit low-grade heating of water and buildings, although there are large variations with latitude and season. At low latitudes, simple mirror devices can concentrate solar energy sufficiently for cooking and even for driving steam turbines.
The energy of light shifts electrons in some semiconducting materials. This photovoltaic effect is capable of large-scale electricity generation. However, the present low efficiency of solar PV cells demands very large areas to supply electricity demands.
Direct use of solar energy is the only renewable means capable of ultimately supplanting current global energy supply from non-renewable sources, but at the expense of a land area of at least half a million km2.
Acknowledgements
Course image: Argonne National Laborator in Flickr made available under Creative Commons Attribution-NonCommercial-ShareAlike 2.0 Licence.
Grateful acknowledgement is made to the following sources for permission to reproduce material in this course:
Except for third party materials and otherwise stated (see terms and conditions), this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence
Figure 4a Andrew Woodley/Alamy
Figure 4b Tony Craddock/Science Photo Library
© Andrew Woodley/Alamy
All other material contained within this course originated at the Open University
This resource was created by the Open University and released in OpenLearn as part of the 'C-change in GEES' project exploring the open licensing of climate change and sustainability resources in the Geography, Earth and Environmental Sciences. The C-change in GEES project was funded by HEFCE as part of the JISC/HE Academy UKOER programme and coordinated by the GEES Subject Centre.
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
Copyright © 2016 The Open University