Animals at the extremes: Polar biology
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Friday, 26 April 2024, 9:00 PM
Animals at the extremes: Polar biology
Introduction
This course is the third in a series of three on Animals at the extreme. In order to get the most from it you should have previously studiedAnimals at the extreme: the desert environment (S324_1)andAnimals at the extreme: hibernation and torpor
This OpenLearn course provides a sample of Level 3 study in Science.
Learning outcomes
After studying this course, you should be able to:
define and use, or recognise definitions and applications of each of the bold terms
outline the special features of the polar regions as a habitat and list some contrasts between the Arctic and the Antarctic
describe some effects of daylength on feeding, fat deposition and reproduction in arctic animals
explain why the environmental controls of appetite, activity level and fecundity are essential adaptations to living at high latitudes and describe some physiological mechanisms involved
describe some adaptations of fuel metabolism and bone formation to dormancy in bears.
1 Polar biology
1.1 Preamble
This course is about animals' structural and physiological adaptations to living permanently in cold climates; hibernation, a special response to transient or seasonal cold, is described in the OpenLearn course Animals at the extremes: hibernation and torpor (S324_2). Living in a polar climate involves adaptations of many physiological systems: appetite, diet, energy storage and reproductive habits as well as thermoregulation. In many cases, such changes involve ‘ordinary’ physiological mechanisms being pushed to extremes. The study of such physiological adaptations can help us to understand how humans and domestic animals could cope with similar conditions that arise under artificial or pathological conditions. For example, obesity is rare among wild animals, even when food is very plentiful, but in humans, the condition is common and often leads to numerous physiological complications, ranging from susceptibility to diabetes to mechanical damage to legs and feet. Most naturally obese animals occur in cold climates, and there is no evidence that they suffer from the complications of the condition that are observed in people and their domestic livestock. Perhaps we have something to learn about the natural regulation of appetite and the organization and metabolic control of fat from these cold-adapted species that have evolved ways of combining fatness with fitness.
On the evolutionary time-scale, modern polar environments, and hence living species of polar organisms, evolved relatively recently. The study of polar organisms provides the opportunity to study physiological adaptations of quite recent origin that evolved in organisms which were already complex and well-integrated. Such changes are comparable to artificial evolution in domestic animals, whether by manipulation of the genome (i.e. intensive artificial selection, gene transfer, etc.), or by drastically altering the diet and husbandry conditions. Polar organisms may help us to under-stand the physiological and psychological implications of the rapid, often drastic changes that we impose upon our own lives and those of our domestic animals.
Antarctica has been isolated from other continents since the Mesozoic supercontinent Gondwanaland broke up and the fragments that became India, Australia and New Zealand drifted away. The rich fossil record in Antarctica shows that a diverse tropical fauna, including early eutherian and metatherian mammals, once lived there. As the continent became colder, many species disappeared and adaptations to the climate evolved in situ in surviving lineages over many millions of years. Consequently, many of the organisms of Antarctica and the surrounding oceans are endemic.
In contrast, much of the Arctic is a large ocean, connected to the Pacific Ocean by the Bering Strait (that became a land bridge several times during the last million years) and through wider channels to the north Atlantic Ocean.
When used as an adjective, ‘arctic’ generally refers to the regions around both Poles and does not have a capital letter. ‘Arctic’ and ‘Antarctic’ are the northern and southern arctic regions, respectively, and do have capital letters. To avoid confusion, the term ‘polar’ is used to mean both arctic and antarctic.
Prevailing winds and deep currents bring plenty of mineral nutrients to the Southern Ocean but the Arctic Ocean, particularly the areas north of Siberia, Alaska and Canada, is nutrient poor. Consequently, the Southern Ocean supports a much greater abundance of marine life than is found in most of the Arctic, except in a few areas such as the Barents Sea around northern Norway and northwest Russia.
Biological evolution in the Arctic has been much affected by the Pleistocene ice age, which produced several periods of glaciation over much of the Northern Hemisphere that began about a million years ago and continued until as recently as 10000 years ago. There were ice ages in the Palaeozoic and early Mesozoic, but until the Quaternary ice age began about 1 Ma ago, the climate had been mild, often warm, over the whole globe for the previous 250 Ma. The climate became colder and drier, promoting rapid evolution in many different lineages of animals and plants. Many species became extinct, but others, particularly descendants of cold-adapted organisms that lived on high mountains, adapted to the new conditions: numerous modifications of the skin and fur, endocrine mechanisms and behaviour and circulatory, respiratory, digestive and excretory systems evolved in many different species over a comparatively short period. Among them was an almost hairless primate, Homo, which adapted successfully to the cold climate in Europe and northern Asia after several million years of evolution in tropical Africa. Many such cold-adapted species ranged over much of the Northern Hemisphere until the climate became warmer during the interglacial period of the last 10 000 years, since when most have been confined to the Arctic.
1.2 The polar environment
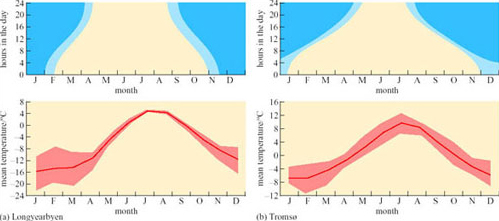
At high latitudes, the Sun's rays always strike the Earth at a large angle from the vertical so they travel through a thicker layer of atmosphere and are attenuated by the time they reach the ground. Because the Earth's axis of rotation is inclined to its path around the Sun, there are large seasonal changes in daylength and the Sun is continuously below the horizon for a period in winter and continuously above the horizon for an equivalent period in summer. The annual changes in daylength and average temperature recorded just inside the Arctic Circle (at Tromsø, Norway) and far into the Circle (at Longyearbyen on the island of Spitsbergen, Svalbard Archipelago) are summarized in Figure 1. The range of annual temperature change is much greater at the higher latitude, and in mid-winter (January and February), the range about the mean is more than 12° C. In polar climates, the temperature can change abruptly and often unpredictably. In fact, both the localities featured on Figure 1 are on coasts, where the sea keeps the climate much more equable. Further inland, fluctuations in temperature are even greater. Polar organisms are thus adapted both to the extreme cold and to abrupt fluctuations in temperature.
The Arctic Circle (66° 30′N), and the equivalent latitude in the Southern Hemisphere, are defined as the latitude above which the Sun is continuously below the horizon for at least 1 day each year. Warm, moist air from the temperate zone rarely reaches high latitudes, so in most polar areas precipitation is low. Much of the water is locked away as ice, which has a low vapour pressure, and the air is very dry (often as dry as a tropical desert) and ground water is inaccessible to plants as well as to animals.
Terrestrial environments in the Arctic are, by geological standards, relatively new, most of the land having been completely covered with a thick layer of ice as recently as 10 000 years ago. Consequently, the soil is thin and fragile, and poor in organic nutrients. The optimum temperatures for plant growth do not coincide exactly with peak sunshine. At Longyearbyen, continuous daylight begins in late April, but the mean temperature does not rise above 0° C (and so the snow and ice do not melt) for another 2 months (Figure 1).
These circumstances, combined with the severe climate, mean that the growing season for plants is short but intensive, and total productivity on land is low, producing little food and still less shelter for animals. Consequently, relatively few species of terrestrial organisms live permanently at high latitudes. For example, although the land area of Svalbard is about 62 000 km2, almost half that of England, there are only a few hundred species of insects and other invertebrates, two resident terrestrial mammals, the arctic fox (Figure 2a) and reindeer (Figure 2b), one bird (an endemic species of ptarmigan) and no reptiles, amphibians or completely freshwater fish. However, many other species spend part of the year on or near the land, often while breeding or moulting: seasonal visitors include more than 30 species of migratory birds (various kinds of geese, auks, puffins, skuas, terns, gulls, and eider ducks and snow buntings), and mammals that feed in the sea, such as polar bears, walruses and several species of seal. The simple ecosystem on land and the severe, erratic climate tend to produce ‘cycles’ of population abundance followed by mass mortality or migration (e.g. lemmings in Scandinavia and Russia). Interesting physiological and behavioural adaptations to these fluctuations in food supply have evolved in some of the larger animals. The vast continent of Antarctica has no indigenous terrestrial vertebrates, although many birds, including penguins, skuas, terns and gulls, and six species of seal spend time on or near land.

Only two species of terrestrial mammal occur naturally throughout the year on Svalbard (although a few others have been introduced by humans during the past century). Figure 2a shows the arctic fox (Alopex lagopus), which also occurs throughout the Arctic, and in mountains at lower latitudes. The picture above, taken in late autumn, shows an adult in its long, dense winter coat. The summer coat is usually greyish brown, often with white markings. Alopex is bred in captivity for its fur, which can vary in colour from grey to bluish in winter, and chocolate brown to fawn in summer, hence the common names, silver fox or blue fox. Figure 2b shows a subspecies of reindeer (Rangifer tarandus platyrhynchus) that is endemic to Svalbard. This picture was taken in July, when the vegetation is at its highest, and these young males are growing antlers for the mating season in September
The situation in the sea is very different. Seawater freezes at −1.9° C, but because of the anomalous relationship between the density and temperature of water, ice floats, insulating the water underneath from the cold air above. Except in very shallow areas, the sea-ice does not extend to the sea-bed, even at the North Pole. Storms and currents sometimes break up the ice, creating many temporary, and some permanent, areas of open water even at high latitudes in mid-winter. Such turbulence also oxygenates the water and admits more light, making the environment much more hospitable to larger organisms.
The movements of ocean currents are complex (and may change erratically from year to year), often resulting in an upwelling of deep water rich in nutrients and promoting high primary productivity in the sea. In most arctic regions, the sea is both warmer and more productive than the land, so at high latitudes there are many more organisms in the sea than on land, at least during the brief summer, and, as in the case of the baleen and sperm whales, some are very large. Some groups of animals, such as bears, that are terrestrial in the temperate zone, have evolved adaptations that enable them to feed from the sea in the Arctic.
Sea-ice is less compact than freshwater ice, and contains many tiny channels containing liquid water as well as cracks caused by weather and currents. Hence sea-ice appears opaque rather than transparent like freshwater ice. The pores harbour a variety of single-celled algae, bacteria and other microbes that form the basis of surprisingly productive food chains. Most of those living on or near the surface are photosynthetic, and during the summer, such microbes are dense enough to confer a brown colour on the underside of the sea-ice. These organisms, and similar ones living on snow and in cold, dry terrestrial habitats, are collectively known as psychrophiles (ψυχρoσ, psychros=cold, Φιλoσ, philos=friend). The continent of Antarctica is generally much colder at comparable seasons and latitudes than most of the Arctic, with the possible exception of large landmasses such as Siberia, Alaska and some of the bigger islands off the north coast of Canada. With its harsher climate, and longer period of biological isolation, Antarctica has a wider variety of endemic, impressively adapted psychrophiles than most of the Arctic.
2 Environmental regulation of physiological processes
2.1 Nutrient budgeting
All plants and animals respond to environmental changes such as the light–dark cycle and temperature, but the impact of the environment on essential physiological processes such as eating, fattening and breeding is more evident and often more finely controlled in polar species than in those that are native to warmer and more equable habitats. Large effects are nearly always easier to quantify and to investigate experimentally, so arctic species offer an excellent opportunity to study the subtle but often important action of environmental changes on physiological processes.
A good place to begin such an analysis is with nutrient budgeting. Energy is expended in the search for food, and in ingesting and digesting it. If food is so scarce that searching is inefficient, or its nutrient content so low that little nourishment is obtained from it, animals may be able to save energy by suppressing appetite and fasting. In polar environments, food is widely scattered both in space and in time. Consequently, the physiological mechanisms that regulate appetite and energy storage are sophisticated and effective in arctic species. Herbivorous animals such as reindeer are directly dependent upon plant productivity and synchronize their foraging and other energetically expensive activities, such as mating and breeding, with it. Daylength (photoperiod) is a more reliable indicator of season than temperature (see Figure 1) and is often an important regulator of physiological mechanisms.
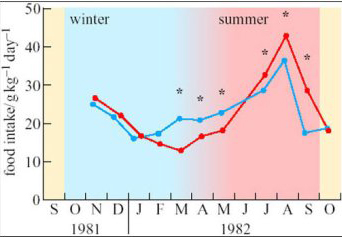
To investigate seasonal changes in the behaviour and metabolism of species native to the high Arctic, a few adults of the subspecies of reindeer that is endemic to Svalbard (Rangifer tarandus platyrhynchus, see Figure 2b) were transported to northern Norway and kept in small outdoor pens there, alongside similar individuals of the native subspecies, Rangifer tarandus tarandus (Larsen et al., 1985). All the animals had continuous, unrestricted access to forage but, as shown on Figure 3, the Svalbard reindeer ate three times as much food in August as in March.
SAQ 1
Are these seasonal changes in the appetite of Svalbard reindeer simply a direct response to the environment?
Answer
No. There were seasonal changes in the food eaten by local Norwegian reindeer as well, but they were less pronounced than those of the animals native to high latitudes. In addition, the largest differences between the two subspecies were observed in mid-March and mid-September, around the equinoxes when day and night are equal in length over the whole globe.
SAQ 2
Do seasonal differences in energy expenditure explain these data?
Answer
No. Being confined in small pens, the reindeer took little exercise all the time. Energy expended on thermoregulation should be greater in cold weather, so if thermogenesis was important, one would expect them to eat more, not less, in the winter.
Reindeer (Figure 2b) grow thick coats of long, hollow hair that insulates the warm skin so effectively that snow accumulates on their backs without melting. Energy expenditure on shivering or other forms of thermogenesis seems to be minimal even in the coldest weather. Foraging is slower and less efficient in winter, and the lower total daily intake is supplemented by utilization of the fat reserves built up during the brief summer, when they eat almost continuously. However, as these experiments show, the seasonal changes in food intake arise primarily from the endogenous control of appetite, and are not imposed upon the animals by food availability. The fine control of appetite is slightly different in subspecies adapted to different climates. The investigators also found small but significant differences at certain times of year between Norwegian and Svalbard reindeer in the rates of lipogenesis measured in adipocytes in vitro, and in the responses of adipose tissue to hormones such as adrenalin.
Metabolic rate, food intake and other aspects of energy balance also change seasonally in birds and mammals that are native to high latitudes. The red, or common, fox (Vulpes vulpes) occurs throughout Europe and northern Asia except in high mountains and arctic regions, where it is replaced by the smaller arctic fox, shown in Figure 2a. As shown on Figure 4a, at above 10° C, the fox's basal metabolic rate (BMR) is about the same in summer and winter, but as the temperature falls, the rise in BMR is delayed and is slower in winter-adapted animals than in those caught in summer.
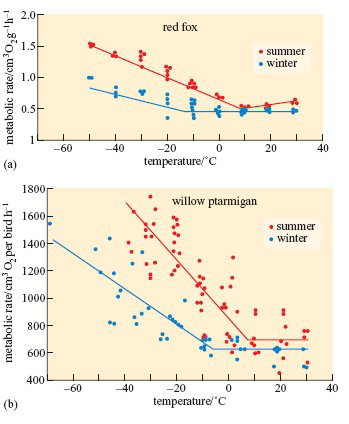
Such phenomena have been intensively investigated in ptarmigan (Figure 5) which are non-migratory, mainly ground-dwelling grouse-like birds that eat twigs, shoots and other plant material. There are two species in Scandinavia and Russia: the willow ptarmigan (Lagopus lagopus lagopus;Figure 5) and the rock ptarmigan (L. mutus mutus). (‘Lagopus’ means ‘foot of a hare’ and refers to the feather-covered or fur-covered feet of the ptarmigan and arctic fox, see Figures 2a and 17.)

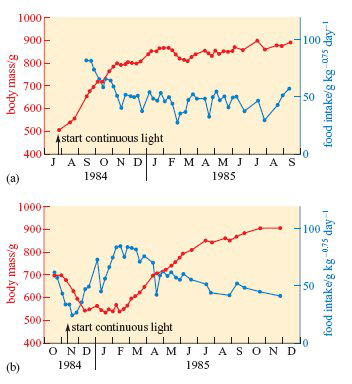
A subspecies of rock ptarmigan occurs only on Svalbard; it is larger than the mainland forms, and has almost pure white plumage during the 8 months of winter. As shown on Figure 4b, the metabolic rate of willow ptarmigan measured at a wide range of temperatures is lower in winter than in summer. The seasonal differences are even greater in Svalbard ptarmigan (L. mutus hyperboreus). Svalbard ptarmigan also eat much more in the late summer than in winter and accumulate fat in the autumn. The experiments summarized in Figure 6 reveal some of the physiological mechanisms that control these changes in appetite and energy storage (Lindgård and Stokkan, 1989).
When exposure to continuous light was started in July (Figure 6a), the birds’ usual autumnal fattening proceeded as normal, but their body mass remained high and food intake fairly low, right through to the following September.
Throughout this period, their plumage remained white and they failed to breed. It was as though the continuous light held them indefinitely in their autumnal condition. However, when exposure to continuous light was started in November (Figure 6b), the birds underwent a complete cycle of changes in body mass and food intake (and began to develop speckled summer plumage) before settling into continuous high body mass and low appetite.
SAQ 3
What do these experiments show about how seasonal changes in appetite and body mass are controlled?
Answer
They are not simply a response to environmental conditions but are at least partly controlled endogenously.
Exactly how such control mechanisms evolved and what happens when animals (or people) are abruptly transported into environments in which their endogenous controls of appetite and energy expenditure are inappropriate are not known.
Although several ruminant mammal species live in mountains and arctic regions (e.g. mountain goats and reindeer, respectively), none is known to hibernate in the strict sense of the term. There are no living species of the family Bovidae smaller than sheep or goats, but some deer (family Cervidae) are less than a tenth of that size as adults. Some tropical deer, notably species of mouse deer Tragulus, weigh only 1–2 kg, well within the range of size of mammals that can become torpid, but none is known to do so.
One reason might be that substantial changes in body temperature would kill the microbes in the rumen that are essential to digestion. Another possibility is that in ruminants, both storage and membrane lipids contain mostly saturated lipids, which have a higher melting point than unsaturated lipids. Laboratory experiments in which animals were fed diets rich in saturated or unsaturated lipids just before hibernation showed that, at least in small rodents, a larger proportion of unsaturated lipids in cell membranes and adipose tissue is essential to successful hibernation . Finally, pregnancy, which lasts a relatively long time in ruminants and usually takes place during the winter, could not be sustained at very low body temperatures.
2.2 Migration for breeding
Birds do not hibernate, but like reindeer, many species undergo daily or seasonal changes in energy expenditure and appetite, and many of the endocrine changes that are an integral part of true hibernation in other groups. The fact that the preliminary stages of hibernation are widespread among vertebrates may help to explain why true hibernation has evolved several times in distantly related taxa. Instead of hibernating, some species of birds migrate to and from breeding areas, where they are able to exploit transient gluts of vegetation or, more often, of the insects and other arthropods that feed on them. Long-distance migratory birds belong a wide variety of taxa, including cranes (order Gruiformes), swifts (order Apodiformes), some swans, ducks and geese (order Anseriformes), cuckoos (order Cuculiformes) and many different kinds of passeriform birds including swallows and martins (family Hirundinidae).
Some birds travel to the Arctic to breed during the polar summer, which can be both cool and short at very high latitudes (e.g. Svalbard), or in regions such as Siberia that have particularly severe climates. It’s worth mentioning in passing that until these remote regions were explored in the 18th and 19th centuries, the breeding sites of many migratory birds were a complete mystery, giving rise to wild speculations about where, if at all, the birds bred. For example, barnacle geese (Branta leucopsis) derive their name from the medieval belief that they arose spontaneously from barnacles. In fact, they breed in Greenland, Svalbard, remote parts of Sweden and northern Russia, with almost all the Svalbard population spending the winter around the Solway Firth and Dumfries and Galloway, Scotland. Red knots (Calidris canutus, order Charadriiformes) are ‘waders’, eating worms, shellfish and other invertebrates collected from beaches, mudflats and estuaries. These small birds (adult body mass about 0.1 kg) form large, dense flocks near sandy or muddy coasts of northern Britain and northwest Europe during the winter (Figure 7). Like many birds, the juveniles eat insects and other small arthropods. Some populations breed between June and August on the Taimyr Peninsula, the most northerly region of Siberia that extends into the Arctic Ocean. The area became free from permanent ice following the end of the last ice age only a few thousand years ago, and is flat and marshy, with several large slow-flowing rivers that support huge populations of mosquitoes and other insects in summer.

SAQ 4
What would be (a) the advantages and (b) the disadvantages of breeding in such places?
Answer
(a) Advantages: fewer predators (though arctic foxes, snowy owls and large gulls such as skuas are present); foods suitable for the chicks and adults are available in large quantities in adjacent habitats; continuous daylight (Figure 1) permits continuous foraging. (b) Disadvantages: the weather is often cold and stormy, and the terrain offers little shelter, so keeping the eggs and chicks warm may pose problems. The breeding season is very short, necessitating rapid growth of the chicks. The journey between Siberia and northwest Europe is tens of thousands of kilometres.
Dutch ornithologists used doubly-labelled water and other techniques to study the growth and metabolism of chicks there, and compared their data to similar observations on other species of the order Charadriiformes with similar habits (sandpipers, dunlins, turnstones, godwits, plovers and oystercatchers) that breed in the temperate climates of northwest Europe (Schekkerman et al., 2003). They found that chicks of the arctic-breeding species both grew faster and generated more body heat, mainly by shivering, than similar birds breeding in temperate climates. The increased thermogenesis was necessary not only because of the severe climate, but also because in Siberia, the parents actually spent less time brooding even very young hatchlings. Red knot chicks are precocious and can forage for themselves at a few days old. They apparently also manage with very little sleep (in sharp contrast to most neonatal birds and mammals, which require many hours of sleep), enabling them and their parents to forage for up to 20 h per day. The total energy expenditure from hatching to fledging was found to be up to 89% higher in the arctic-breeding knots, but the chicks were dependent on the parents for only 17–20 days, a shorter period than related species of similar size.
This study demonstrates a range of far-reaching adaptations of thermoregulation, growth rate and sleep requirements in birds that breed in polar regions. The opportunity to exploit the temporary abundance of food apparently outweighs any disadvantages associated with these adaptations of growth rate and thermogenesis and the energetic costs of migration.
The journey itself requires further metabolic specialization. The birds break their journey at several places where food is abundant and easily obtained. However, since time is short, some stopovers last as little as 1–4 days, during which time they must take on enough fuel for the next stage of the journey. The closely related sandpiper (Calidris mauri) that also breeds in the Arctic can fatten at 0.4 g day−1 (4.5 times the normal rate) during brief stopovers. This remarkably high rate of deposition of fat stores is possible due to a temporary increase in the activity of lipogenic enzymes such as fatty acid synthase.
2.3 Environmental regulation of breeding
As pointed out in Section 1.1, primary plant productivity occurs for only a few months in the summer, so the reproductive physiology of most arctic animals, particularly herbivorous species, is tightly synchronized with the seasons. On Svalbard (Figure 2b), more than 90% of the reindeer fawns are born in the first week of June. The mothers of those born too soon or too late are often unable to find enough food to support lactation and the fawn fails to thrive. As shown on Figure 1, the onset of continuous daylight and that of the conditions that support plant growth are several months out of phase. This situation poses little problem for reindeer, because the duration of pregnancy is almost constant and they mate only during a brief rutting period in September, when the daylength is changing rapidly. But this environmental cue alone would not be an accurate control on the timing of breeding of resident herbivorous birds such as ptarmigan that breed in mid-summer.
The physiological mechanisms that control the timing of several aspects of mating and breeding in the Svalbard ptarmigan (Lagopus mutus hyperboreus) have been investigated in detail (Stokkan et al., 1986). Their plumage is almost pure white in winter but speckled brown feathers appear in summer and the adult males have a red fleshy ‘comb’ over each eye. Figure 8 shows the seasonal changes in these secondary sexual characters, the maturation of the gonads, and the concentration of luteinizing hormone (LH) in blood plasma in ptarmigan shot on Svalbard. LH levels (Figure 8a) are low from August until February, when the Sun reappears (see Figure 1). The blood plasma LH levels and body mass (see Figure 6) start to increase slowly, and in March first primary, then secondary, spermatocytes appear in the testes (Figure 8b) and the combs begin to grow (Figure 8c). However, there are no mature spermatozoa until the end of May, so the gonads mature much more slowly than in most other seasonally breeding birds. Pigmented feathers also do not appear until June, just before the snow melts (Figure 8c).
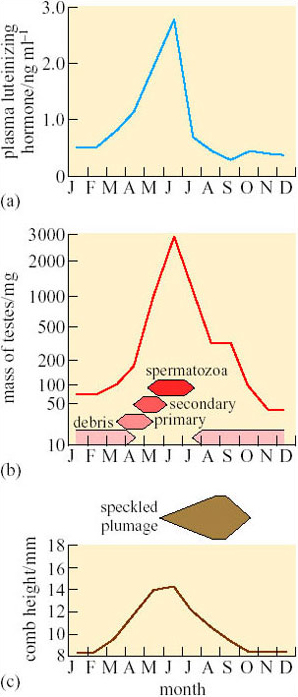
SAQ 5
Are there any advantages in delaying the development of pigmented feathers?
Answer
Speckled plumage is probably much more conspicuous to potential predators (i.e. arctic foxes) against a background of snow than pure white feathers, so it would be advantageous not to produce the breeding plumage until it is essential for courtship and mating.
Throughout the year, LH levels are lower in female (Figure 9a) than in male (Figure 8a) ptarmigan, but, as in males, there is a sharp peak in June that coincides with maximum mass of the ovary and the period during which eggs are laid (Figure 9b). However, LH is also fairly high in March (Figure 9a), several months before the gonads become active. Some other factor, perhaps non-photoperiodic inhibitory input from the environment (e.g. cold weather), must be delaying the maturation of the ovary.
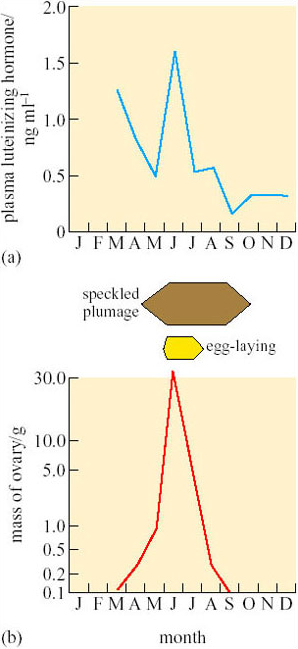
2.4 Variable fecundity
The food supply for most polar species depends on several unpredictable factors so successful breeding is far from certain, even if births are tightly synchronized with the seasons. Maintaining pregnancy and feeding the offspring after birth (or hatching in birds) are energetically expensive. The death of the offspring before its maturity represents an irredeemable loss of ‘reproductive investment’ for the parents, particularly the mother, although the earlier in parental nurturing that the death occurs, the smaller the loss to the parents. Various mechanisms of environmental determination of fecundity have evolved among large birds and mammals and are particularly evident in arctic species.
Like most large ungulates, reindeer produce only one offspring a year and suckle it for more than 6 months, by which time the next pregnancy may be well underway. Observations on Svalbard reindeer show that in December, nearly all adult females are pregnant, having conceived during the mating season in the previous September. But as winter progresses, the proportion that are pregnant falls, and by June the following year, any fraction from over 90% to less than 10% of the adult females give birth to a fawn. The other pregnancies must have ended in abortion or reabsorption of the fetus. In each year, the proportion giving birth is approximately the same in all areas of Svalbard that can be studied, suggesting that it is related to the climate. Exactly how the reindeer ‘knows’ when to terminate a pregnancy which she is unlikely to be able to complete successfully is currently under investigation, but the quality or quantity of the food available during the winter is the most likely factor.
The fecundity of arctic foxes is also very variable: in years when prey and carrion are abundant, some litters consist of as many as 20 pups (with an average of 10–12 in Canada and 6.4 on Svalbard), a very large number for a canid (dog-like) mammal, but very few breed at all in years when food is scarce. A similar pattern is found in predatory birds such as the snowy owl, which also feeds on rodents and hares that undergo population cycles. When prey are abundant, the fox or owl parents can raise a large number of pups or chicks but if food availability suddenly falls (due to mass mortality or migration of prey or a change in the weather), most or all of the offspring may starve in the nest. Food intake and/or energy stores somehow regulate ovulation and/or the number of ova that implant successfully and develop, so that as far as possible, fecundity is adjusted to food supply in a fluctuating environment. However, very little is known about the mechanisms involved: the formation and maintenance of the placenta depend upon several different hormones, some of them secreted from the pituitary and brain. The body may respond to stress or insufficient nutrition by terminating the pregnancy prematurely, thereby enabling the mother to build up reserves that could support a pregnancy in the next breeding season at which the prospects of a more successful outcome appear brighter.
2.4.1 Summary of Sections 1 and 2
Large seasonal changes in temperature and sunlight dominate primary plant production and hence the food supply. Food intake is regulated by the endogenous seasonal control of appetite, fattening and activity, as well as by food availability. Energetically demanding activities such as breeding and migration are only feasible during a brief period and must be tightly synchronized to season. Greater accessibility of food suitable for chicks makes long-distance migration to and from high arctic regions worthwhile for some birds. Adaptations of thermoregulation and growth rate enable breeding to be completed during the brief summer. Arctic ecosystems involve relatively few species, some of which are prone to abrupt, cyclic changes in population abundance, so food supplies change erratically from year to year and from place to place. Most physiological adaptations to these features of the polar environment probably arise from modification and refinement of mechanisms that occur in temperate-zone species.
3 Natural feasting and fasting
3.1 Introduction
It is clear from Sections 1 and 2 that seasonal or irregular periods of fasting are an integral part of living at high latitudes, especially for large animals. When people (and many tropical and temperate-zone mammals) lose weight, either because they are eating less or because they are suffering from a digestive or metabolic disorder, protein is broken down in substantial quantities long before the lipid stores are exhausted. Even frequent and vigorous exercise cannot prevent the breakdown of lean tissue, although it can often reduce or delay the process, particularly in young people. The loss of protein causes muscles to become weak and wasted, and the skin and hair to appear shabby. Immune function is also impaired, weakening resistance to parasites and infectious diseases. These undesirable side-effects of fasting do not normally afflict mammals and birds that naturally go without food while remaining active for long periods.
3.2 Penguins
Penguins (order Sphenisciformes) are an ancient and distinctive group of flightless, short-legged birds that evolved in the Southern Hemisphere, probably around New Zealand, about 65 Ma ago in the late Cretaceous, although the oldest known fossils date from about 45 Ma ago.
At a maximum body mass of more than 40 kg, the emperor penguin (Aptenodytes forsteri;Figure 10a) is the largest living penguin (some fossil species were much bigger) and is found further south than any other vertebrate. Like other penguins, emperors feed on fish, squid and large crustaceans that they catch by diving and chasing the prey underwater. The main predator of adult penguins is the leopard seals (Hydrurga leptonyx;Figure 10b), the largest and most agile antarctic seal, that has a varied diet including other seabirds and smaller seals, as well as fish, squid and crustaceans.
Emperor penguins breed on the iceshelf, away from predators such as skuas that take eggs and chicks, on breeding grounds that may be as far as several hundred kilometres from the open water. The males leave the feeding areas in early April (autumn in Antarctica) and fast during 6 weeks of courtship and for a further 2 months while brooding. Only one egg is laid, and the male carries it on his feet and broods it in a special flap of feathered skin that extends from his abdomen. Brooding penguins are inactive, keeping close together in large groups and walking an average of only 30 metres per day, thereby minimizing energy expenditure to near BMR. If his mate has not returned by the time the chick hatches, the male feeds his offspring on ‘curds’ formed from deciduous tissue in the oesophagus and broods it as he did the egg (Figure 10a). As soon as he is relieved by his mate, he walks back to the open water in what is by then midwinter, continuously dark and very cold.

The female also fasts during courtship, but she returns to the sea after presenting her mate with a single egg that is large relative to her own size. The female fattens quickly while at sea, eating 6–8 kg per day and increasing her body mass by about one-third, before returning to the breeding grounds to take her turn to feed the chick on curds and partially digested food regurgitated from her stomach.
René Groscolas and other French biologists from Strasbourg spent many months in Antarctica studying the physiological mechanisms behind these habits (Groscolas, 1982, 1986). Figure 11 shows the measurements that they made on wild penguins during the breeding season and in the following 3 weeks, while the birds were artificially prevented from returning to the sea to feed at the end of the natural fasting period. Every few days, marked penguins were caught, weighed, their rectal temperature measured, and a sample of venous blood taken.
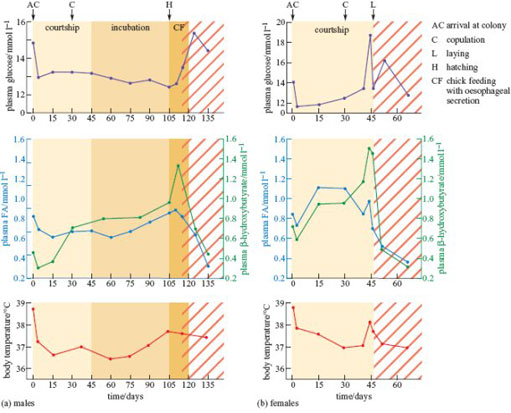
While fasting at the breeding colony, the mean body mass of the males fell by 40.5%, from 38.2kg to 22.75 kg, at an average rate of 35 g per day. After falling slightly during the first few days, the body temperature, and levels of glucose and fatty acids in the blood plasma were constant (Figure 11a), and well within the ranges of values measured in penguins that were feeding regularly. The ketone β-hydroxybutyrate is produced by partial oxidation of fatty acids and can substitute for glucose in some energy-producing pathways in some tissues. Its concentration increased steadily, reaching a peak when the fathers began to feed their chicks. The smaller females lost only about 22% of their initial body mass during their shorter fast. Except during the period of egg-laying, the pattern of changes is similar to that of the males.
SAQ 6
Why should egg-laying affect body temperature and metabolism?
Answer
Egg production involves the synthesis of large quantities of protein and lipid (for yolk), and the withdrawal of calcium stores (for shell formation), which generate heat and require levels of circulating glucose similar to those that support strenuous activity.
The tenfold increase in the concentrations of β-hydroxybutyrate is small compared to changes of up to 40-fold observed in the blood of pigeons, poultry and humans after just a few days of starvation. When artificially prevented from returning to the sea at the end of their normal fast, plasma fatty acid and β-hydroxybutyrate concentrations decreased sharply in penguins of both sexes. Their rate of weight loss also increased abruptly, reaching a mean of 542 g per day for the lightest penguins that weighed only 17.5 kg.
SAQ 7
What can you deduce from these observations about the penguins' fat stores and energy metabolism?
Answer
Production and utilization of free fatty acids decrease, probably because stores of triacylglycerols are almost exhausted. So the penguins start utilizing protein at a much higher rate. Because much less energy is produced from the breakdown of each gram of protein, a much higher rate of weight loss is necessary to meet the energy requirements of maintaining an almost constant body temperature.
This conclusion is confirmed by increased excretion of uric acid during enforced starvation. Other measurements indicate that during natural fasting, 93% of the penguins' energy comes from oxidation of fatty acids released from adipose tissue (Figure 12). The small quantity of glucose needed to support glucose-dependent tissues (e.g. the brain) is formed mainly from the glycerol in triacylglycerols, and only small quantities of protein are utilized.
SAQ 8
Are the reserves replenished in the same way as they are depleted?
Answer
No. As shown on Figure 12, protein is withdrawn last during fasting but replenished more rapidly than lipid when the penguins start feeding again.
These observations suggest that, as in other animals, loss of protein has serious disadvantages and is only a ‘last resort’ used when other energy reserves are exhausted.
The mean body mass of male penguins leaving the colony is around 23 kg, which, from calculations based upon the data in Figures 11 and 12, indicates triacylglycerol reserves of about 2 kg. This amount is just sufficient to sustain the penguin as it walks, using energy at 2.8–4.5 times BMR, as far as 100 km back to the open sea. Under normal circumstances, the birds begin feeding just before exhausting completely the lipid in their adipose tissue (Figure 12). Utilization of protein reserves involves drastic alterations in metabolism and they do not last long, so if the weather is unusually severe, or the sea-ice is exceptionally extensive, or stocks of fish at the feeding grounds are low, penguins that were even slightly underweight at the start of the breeding season may not survive. Indeed, Groscolas suggested that the decrease in the plasma concentration of fatty acids and/or of β-hydroxybutyrate may be the metabolic signal (the black arrow on Figure 12) that prompts the parent to abandon its chick and return to the sea, even if its mate has not yet come back. Each year around 30% of eggs and chicks are abandoned for various reasons, and without parental care they always die. However, mortality among adult penguins is quite low, and each bird may breed many times during a long lifetime.
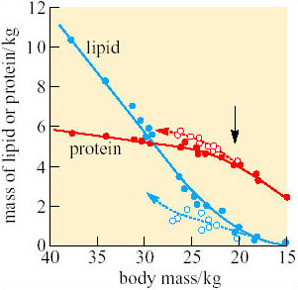
Comparison between different species of penguin shows that, in general, larger species can fast for longer, suggesting that the very large extinct penguins may have undergone fasts lasting many months.
3.3 Bears
Brown or grizzly bears (Ursus arctos), and black bears (U. americanus) feed throughout the summer on grass, fruit, nuts, fish, small mammalian prey and carrion. In autumn, all brown and black bears fatten rapidly before entering caves or hollow trees where they become dormant for weeks or months. The terms ‘hibernation’ and ‘torpor’ are sometimes used to describe this state in bears. To avoid confusion with true hibernation, this phenomenon is here called ‘dormancy’. Much of the research on the metabolic basis of this physiological state has been carried out in the USA on the black bear, which occurs over most of USA and southern Canada and is smaller and easier to maintain in captivity than brown bears or polar bears.
3.3.1 Dormancy in black and brown bears
The dormant state of bears differs from true hibernation in that the body temperature does not fall below 31–35° C and a major disturbance (such as an intruding biologist) can arouse them to full activity in a few minutes. Dormant bears do not eat, drink, urinate or defaecate, the heart rate drops from 50–60 beats min−1 to 8–12 beats min−1, and oxygen consumption is only 32% of that of actively foraging bears. Nonetheless, the rate of protein turnover, as measured by the rate of dilution of 14C-labelled amino acids injected into the blood, is three to five times higher during dormancy than in normal activity. Protein synthesis, particularly of enzymes involved in lipid and protein metabolism, also continues unabated during dormancy. The degradation of proteins to urea, however, is greatly slowed in dormancy. In these respects, the reciprocal changes in protein metabolism of the hibernating bear resemble those of humans and other mammals of tropical origin living on protein-deficient diets: essential amino acids are incorporated into proteins in the liver, but oxidation of amino acids and excretion of nitrogen are greatly reduced.
A small but significant quantity of urea is produced throughout dormancy but it is not excreted. Instead, it passes across the gut epithelium and into the lumen, where it is further degraded to ammonia (NH3) and carbon dioxide by the gut bacteria. The carbon dioxide is excreted with the respiratory gases, but the fate of the highly soluble, and in high concentrations toxic, ammonium ions (NH4+) is more interesting. In dormant bears, the blood concentrations of amino acids, total protein, urea and uric acid during dormancy are similar to those of active bears that are feeding regularly. Since there is almost no net elimination of the nitrogen, it must be re-incorporated into amino acids. The most important source of carbon for this process is glycerol. If 14C-labelled glycerol is injected into a dormant bear, the label quickly appears in alanine, then in other amino acids, and finally in plasma proteins.
SAQ 9
Where would the glycerol come from normally in a dormant bear?
Answer
Glycerol is produced from lipolysis of triacylglycerols.
The fatty acids released by lipolysis are used in energy metabolism, but much of the glycerol (that in other mammals is mainly oxidized) is combined with ammonia to form amino acids, which are incorporated into proteins in the normal way. This mechanism recycles the nitrogen so efficiently that the concentration of urea in the blood actually decreases slightly after several weeks of dormancy.
The rate of excretion of nitrogen can be estimated as the ratio of the concentrations of urea (U) to creatinine (C) in the blood (U:C ratio). Malcolm Ramsay and colleagues measured the U:C ratio in blood samples collected from wild polar bears in northern Canada (Ramsay et al., 1991). Creatinine is formed from the breakdown in muscle of phosphocreatine, a high energy phosphate compound, and is a minor but constant source of excreted nitrogen. In bears, the concentration of creatinine in blood plasma increased about threefold during the first 1–2 days in dormancy and then remained constant. The U:C ratio is around 50 in most mammals, especially carnivores that are eating regularly, and does not normally fall lower than 25, even during prolonged fasting. But U:C ratios of less than 10 are frequently measured in dormant black bears, indicating that during dormancy a high proportion of the urea is re-incorporated into proteins instead of being excreted (Nelson et al., 1984). Consequently, the bears’ lean body mass is hardly diminished even after months of dormancy and their muscle strength is unimpaired.
In starving humans and most other fasting animals, β-hydroxybutyrate and acetoacetate (ketone bodies) are formed by partial oxidation of fatty acids (see Figure 11). They are normally eliminated by further oxidation, but sometimes the presence of a high concentration of ketones disturbs the acid-base buffering of the blood and a comatose state called ketosis develops. In many hibernatory mammals, very high concentrations of ketone bodies trigger arousal. Ketone bodies increase in dormant bears as well but only to a maximum of ninefold between normal activity and dormancy and the toxic effects of ketosis have never been observed. Experiments in which labelled glycerol is injected into the blood of dormant bears show that, as well as being incorporated into amino acids, substantial amounts of labelled glycerol also appear in triacylglycerols.
SAQ 10
What does this observation show?
Answer
It shows that, as well as lipolysis of lipids stored in adipose tissue, resynthesis of triacylglycerols from fatty acids and glycerol is also occurring at a significant rate. The rate of triacylglycerol turnover may be higher during dormancy than during normal activity, and may limit the rate at which free fatty acids can enter the pathways that produce β-hydroxybutyrate and acetoacetate, thereby preventing ketosis and enabling the bears to sleep undisturbed for long periods.
SAQ 11
Are there any other metabolic advantages of utilizing fat during dormancy?
Answer
Oxidation of fat produces water. Since the bears do not drink during dormancy (except perhaps the occasional mouthful of snow), and the surrounding air is very dry, such metabolic water probably makes a significant contribution to water balance. Total body water, blood volume and the water content of red cells and plasma remain normal during dormancy, indicating that the water generated by such metabolism is indeed sufficient to offset the small losses due to respiration of the dry, cold air. Thus the large quantities of adipose tissue triacylglycerols in bears are much more than just an energy store: they are central to the bears’ metabolic adaptations to dormancy.
Measurements of composition of the respiratory gases reveal that the respiratory exchange ratio (RER) falls from 0.78 when the bears are fully active to 0.62–0.69 during dormancy. Such values are exceptionally low: the normal minimum RER for mammals, representing oxidation of lipid only, is 0.71. The low RER shows that some of the carbon dioxide that would normally be excreted through the lungs fails to appear. Carbon dioxide cannot be stored in significant quantities because as hydrogen carbonate (HCO3–), it alters the acid-base balance of body fluids, so it must be converted into non-volatile compounds, possibly by the microbes in the gut or by enzymes in the bear's liver.
Like other metabolic processes, the urea cycle and protein synthesis generate quite a lot of heat. The high rate of these processes during dormancy, together with the bears’ large size and thick, insulating fur, combine to maintain a much higher body temperature than that of small mammals in deep hibernation. Fully functional brown adipose tissue has not been demonstrated in bears, even in neonates, although small areas of white adipose tissue have some structural features that resemble those of BAT. Nonetheless, at a body mass of less than 1 kg, bears are smaller at birth, relative to the size of their parents, than any other eutherian mammal, and they are born in mid-winter or early spring.
Bears become fully active, eating and able to deal with predators within hours of leaving the den after weeks of dormancy. Astronauts after long periods in space and people recovering from illness or injury cannot do the same: especially in the elderly, the skeleton is weakened by more than a few days of immobilization as bone is reabsorbed. Vertebrae or limb bones may fracture under very weak forces; sometimes just standing is sufficient to cause injury. Measurements on people resuming activity after a period of bedrest show that the rate of reformation of bone is 2–3 times slower than its loss during immobilization. How to bears manage to avoid similar problems during and immediately after dormancy?
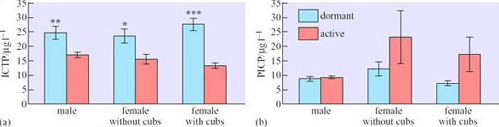
Carboxy-terminal propeptide of type 1 collagen (PICP) is the remnant of nascent type 1 collagen (the principal protein in bone) that is cleaved off by proteases as the protein is incorporated into bone. It can be measured in the blood serum, and thus serves as a convenient marker of bone formation. Another serum protein, carboxy-terminal cross-linked telopeptide of type 1 procollagen (ICTP), acts as a marker of bone resorption. Figure 13 shows some measurements of these proteins in breeding and non-breeding black bears (Ursus americanus) during dormancy and when actively feeding in the wild in the mountainous region of Virginia, USA (Donahue et al., 2003).
SAQ 12
What conclusions about bone loss and reformation can you draw from Figure 13?
Answer
No statistically significant differences between dormant and active bears were found in the measurements of PICP, though there was a trend towards higher values for active females. In contrast, ICTP, especially in females who were feeding cubs, was higher while they were in the den.
SAQ 13
Do the data in Figure 13 explain how bears avoid increased risk of bone fractures just after emerging from the den after a period of dormancy?
Answer
No, not fully. Bone loss (Figure 13a) is still higher during dormancy (though the differences between measurements from bears found in dens and full activity are not as great as between sedentary and exercising people or rats). But the data provide no evidence that bone formation is consistently greater while the bears are active.
SAQ 14
Could the investigation have failed to reveal important aspects of the time course of bone turnover during dormancy and activity?
Answer
Yes. This investigation would fail to detect any brief period of acceleration of bone formation just before or just after emergence from the den. It is important to study the markers of bone turnover during the transitions between dormancy and activity.
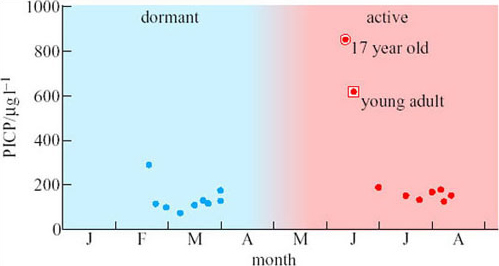
The scientists were able to obtain measurements of the bone formation marker (PICP) before, during and after emergence from a few bears (Figure 14). These measurements show that PICP is four- to fivefold higher early in the remobilization period compared with mid-summer. This brief peak in the concentration of PICP may indicate that bone formation increases just when it is most needed.
SAQ 15
Do the data in Figure 14 indicate exactly how long the peak concentration of PICP in bears emerging from dormancy lasts?
Answer
No. Unfortunately, no bears were found and tranquilized between the end of March and the beginning of June, so we have no information about this crucial period. Such are the problems of studying wild animals under natural conditions.
This more efficient compensatory mechanism for recovering from immobilization-induced bone loss, combined with low bone loss during disuse, enables bears to switch abruptly between dormancy and full activity. The control mechanisms that prompt the rapid increase in bone formation remain to be explored. Its elucidation might enable physicians to induce similar effects in people recovering from hip-replacement operations and other situations in which long periods of immobilization are unavoidable.
SAQ 16
Why are the differences between the values for dormancy and activity greatest for females that are suckling cubs (Figure 13)?
Answer
Lactating females are transferring some of the minerals (especially calcium) in the bones to the milk (where it is incorporated into the cubs’ skeletons), further weakening their own skeleton. Breeding females thus need to maximize bone reformation after dormancy.
3.4 Bears (continued)
3.4.1 Fasting in polar bears
Polar bears (U. maritimus) are almost entirely carnivorous and are the only living species of bear to obtain almost all their food from the sea. Their main prey is ringed seals (Phoca hispida), both adults and suckling pups; they catch the adults when they come up to breathe through holes in the ice, though detailed observations show that as few as one in five attempts results in a kill. The seals are born in holes in the snow, and their mothers leave them hidden there while they go to feed, returning to suckle them at least once a day. The young seals fatten and grow very rapidly on their mothers’ exceptionally rich milk. Until their fluffy white fur is replaced by the sleek, more waterproof adult coat, the young seals cannot enter the water even if it is accessible because they quickly get too cold. The seal breeding season in spring and early summer thus provides polar bears with plentiful prey.
SAQ 17
What features of the arctic environment enable the seals to maintain their population in spite of predation from bears?
Answer
The seals travel long distances and ice conditions suitable for breeding are not necessarily in the same place each year, so the bears may not find many of the seal breeding colonies.
How often are polar bears successful in finding food? Do they fast when out on the ice, as well as when in dens? Polar bears range over such a wide area of inhospitable terrain that such questions, though vital to the management of the species in the wild, are not easily answered by direct observation. The study of nitrogen metabolism in black bears suggests an indirect way of investigating such topics.
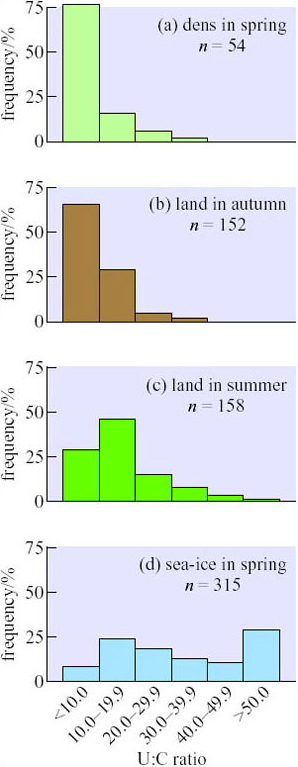
Figure 15 shows some measurement of the ratio of urea to creatinine in blood samples collected from polar bears in northern Canada that were temporarily sedated with drugs injected from a dart gun.
SAQ 18
What do these data suggest about food sources and hunting success in bears?
Answer
More than 75% of bears in dens (Figure 15a) had U:C ratios of 10 or less so they were obviously not eating, but the U:C ratios were 19.9 or lower in 70% of those caught on land in summer and autumn, showing that they were also fasting (Figures 15b and c). Out on the sea-ice in spring (Figure 15d), more than half the bears sampled had U:C ratios of 30 or more, indicating that they were feeding frequently. At least 10% of the bears in this sample were fasting: either they were inexperienced, inefficient or unlucky hunters of seals or (as quite frequently happens) they were forced to give up their kills to larger bears that threatened them. Alternatively, they may be ‘voluntarily’ anorexic while mating: during the spring, large males attend oestrous females closely and may fight with rivals, leaving little time for hunting.
Ice conditions that favour catching adult seals are strongly dependent upon weather and water currents, and so are widely scattered in space and time. Food supply is probably erratic even for the most proficient bears. Seal hunting is almost impossible for several months in the summer and autumn when the sea is unfrozen, and the only food available to polar bears is the odd bit of carrion and a very small quantity of plant food. The data in Figure 15 show that nearly all polar bears fast for long periods in the summer and that, for many, the food supply is unreliable even in winter and spring. Thus polar bears seem to adopt many of the metabolic features of winter dormancy in the omnivorous brown and black bears, while remaining active enough to be able to travel long distances between seal-hunting grounds. They become lethargic and remain inactive for long periods when weather conditions or terrain make hunting impossible, suggesting that they have become ‘dormant’ without actually being asleep in a den.
This theory is confirmed by observations on polar bears held temporarily in captivity. Bears caught in autumn were starved for 5–7 weeks, fed for 3 days, and then fasted again. Blood samples were taken just before and for several days after feeding, and the concentration of urea and creatinine measured. During the imposed fast, the U:C ratios averaged only 11.0, but rose abruptly to 32.0 after feeding and then declined to 22.8.
Polar bears are a relatively new species, almost certainly evolving from brown bears during the last 100 000 years. They must have inherited the capacity for dormancy from their omnivorous ancestors that fed mainly in the summer and autumn. In males and non-breeding females, dormancy takes the form of inactivity during the summer months and in winter, intermittent fasting between widely scattered and irregular feeding opportunities.
Breeding females undergo periods of fasting more closely tied to the seasons. Shortly before giving birth in December or January, the pregnant females migrate to areas where deep snow drifts suitable for making dens have formed against river banks or similar obstacles. Around Alaska, polar bears find denning places on the sea-ice but those around Svalbard and Hudson Bay in Canada travel inland, sometimes substantial distances. The mothers give birth in the snow den and suckle their cubs there for up to 5 months, during which time they remain inactive and do not feed at all, but they are alert and, so far as we know, the body temperature is normal.
Lactation is metabolically very demanding, especially during fasting. The mobilization of lipids, proteins and minerals from storage tissues and the skeleton and the synthesis of milk would produce more than enough heat as a by-product of metabolism to maintain normal body temperature. For obvious practical reasons, there are few physiological data on breeding polar bears. However, indigenous people who traditionally hunted the animals for their meat and skins report that bears in maternity dens are no soft target, and the adult females are quick to put up a fight.
Polar bear cubs are weaned over many months, and while food is scarce, mothers sometimes suckle offspring that are almost as large as themselves. Females never breed more frequently than every other year, and in some areas, the interbirth interval may average more than four years. Although polar bears mate in the late winter, the early embryo undergoes delayed implantation (a phenomenon that seems to be widespread among mammals, especially in carnivores whose food supply is irregular) and gestation does not start until the autumn.
SAQ 19
How could delayed implantation enable female polar bears to adjust reproduction to food supply? How is this adaptation similar to that of reindeer?
Answer
Like the reindeer, female polar bears can adjust their reproduction to the food supply for the particular year and location: fortunate bears may find large, fecund colonies of breeding seals and be able to fatten rapidly by eating several pups a day. Such animals may lay down reserves of nutrients sufficient to raise triplets. Others may produce only one cub, or, if food is really scarce, the pregnancy may be terminated, and the female becomes receptive in the following mating season.
3.5 The structure of adipose tissue
Since food is only available seasonally or intermittently at high latitudes, many arctic birds and mammals, including polar bears, Svalbard reindeer, arctic foxes, seals and walruses, naturally accumulate large stores of fat. The quantity of energy stored and the metabolic control of its use are finely adjusted to the habits and habitat of the species. This section is concerned with the cellular structure and anatomical organization of adipose tissue in such naturally obese species. Most laboratory mammals do not naturally become obese, and must be induced to do so by drastic measures such as changes in diet, drugs or surgery. Although it is impossible to carry out as detailed measurements or carefully controlled experiments on wild animals as it is in the laboratory, arctic species provide a rare opportunity to study fattening and obesity as natural phenomena, rather than as pathological or artificial conditions. Observations on these naturally obese animals can help resolve discrepancies between mechanisms that can be demonstrated experimentally in rats and those that seem to occur in people.
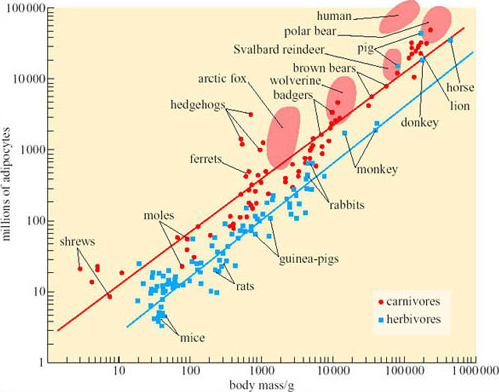
One important aspect of obesity is the contribution of adipocyte enlargement and the formation of additional adipocytes to animals’ increased capacities for storing lipid. In adult rats and mice, fattening is achieved almost entirely by enlargement of adipocytes: the number of cells does not change. The matter is not easy to investigate in humans because there is no really accurate, non-destructive way of measuring total adipocyte complement, but indirect estimates suggest that the accumulation of more adipocytes makes a significant, in some people the dominant, contribution to obesity. In order to establish whether adipocyte proliferation is also essential to expansion of the lipid storage capacity in naturally obese arctic animals, we have to find a way of calculating how many adipocytes would be expected in an animal of any particular body mass. Figure 16 shows some measurements of the numbers of adipocytes in some temperate-zone and tropical mammals (Pond and Mattacks, 1985). The equations for the regression lines drawn on Figure 16 can be used to calculate the number of adipocytes expected in an animal from its body mass. The predicted adipocyte complement can then be compared with the measured adipocyte complement.
Such comparison shows that naturally obese arctic mammals such as polar bears, arctic foxes, wolverines (large mustelid carnivores, related to otters, stoats, weasels, mink, ferrets and badgers) and reindeer have more adipocytes than expected, usually between twice and four times as many, although a few specimens have almost exactly the predicted number of adipocytes. Such proliferation of adipocytes is modest compared to that of humans: some obese people have more than ten times as many adipocytes as expected from comparison with the data in Figure 16.
However, the adipocyte complement of the wild mammals was found to be quite variable in otherwise similar specimens collected from the same area at the same time. Many factors such as hunting ability and appetite determine individual differences in fatness, but among the carnivores there was no evidence that individuals with more adipocytes were normally any fatter than those with fewer adipocytes: the adipose tissue of the former simply consisted of numerous, relatively smaller adipocytes. Individual variation in adipocyte complement is also observed in humans, with some people having relatively few, large adipocytes and others more numerous smaller ones, but it is not as conspicuous in laboratory rats, all of which seem to have about the same adipocyte complement in relation to their body mass unless artificially manipulated to make them unnaturally obese.
SAQ 20
Do these observations suggest that people who have large adipocyte complements are, or will inevitably become, obese?
Answer
No. In the wild carnivores, fatness does not correlate with adipocyte complement. The same may be true of other mammals including people.
We know very little about the origin of such individual differences in adipocyte complement: adipocyte proliferation takes place mainly during the suckling period, and the exact course of growth at this age may differ from one individual bear, arctic fox, wolverine and reindeer to another, depending upon the number of littermates and the amount of food available to its mother.
SAQ 21
Would it be possible to determine the fatness of a particular bear or reindeer by measuring the volume of a sample of its adipocytes?
Answer
No. Adipocyte volume would not be an accurate measure of fatness because the relationship between the total mass of adipose tissue and the volume of its adipocytes would be different in specimens that have large or small adipocyte complements.
Unfortunately, assessment of fatness from biopsy samples of adipose tissue is much more satisfactory in rats (because their adipocyte complement is more constant) than it is in either naturally obese arctic mammals or in humans.
3.5.1 Summary of Section 3
Penguins and many other large polar animals fast for long periods while remaining active and at near-normal body temperature. Emperor penguins fatten before the breeding season and fast for weeks during courtship and reproduction. Very little protein is broken down until lipid stores are nearly exhausted. Energy reserves determine an individual's behaviour such as feeding or abandoning the chick. Omnivorous brown and black bears feed in summer and become dormant in winter: they stop feeding and enter dens, where their metabolism slows and is supported almost entirely by lipids released from adipose tissue. Urea is recycled and very little nitrogenous waste is excreted, so the protein in muscle, liver and other lean tissues is not depleted, as normally happens in prolonged fasting. Bone may be withdrawn from the skeleton during long periods of inactivity, but the tissue is restored to normal strength by rapid deposition of new bone in spring. Similar physiological processes occur in carnivorous polar bears when food is scarce but, except for breeding females, there is no regular, prolonged period of dormancy in a den.
4 Thermal insulation
4.1 Insulation in terrestrial endotherms
For organisms of similar size and shape in a similar thermal gradient, the rate of heat loss from convection is up to 90 times as fast in water as in air, so in temperate climates, aquatic endotherms need much more efficient insulation than terrestrial species. Since seawater freezes at −1.9° C, but the temperature of the air around the Poles can fall below −50° C, the insulation requirements of aquatic and terrestrial polar animals are not very different. Nonetheless, there are important differences in the tissues involved and in their responses to different environments. We start here with a discussion of terrestrial endotherms, then follow this aquatic endotherms and a brief discussion of humans in polar regions.
Relatively minor changes in body shape can contribute much to reducing heat loss. Polar bears have relatively small, round ears, huge, shaggy feet and the tail is much reduced. Svalbard reindeer (Figure 2b) are smaller and stockier, and have shorter ears, legs and snout than subspecies that live further south.
SAQ 22
The continental climates of northern Canada and Russia are as cold or colder than that of most of Svalbard, but the native reindeer nonetheless have long legs. What factors other than minimizing heat loss might determine their body shape?
Answer
Svalbard reindeer have no natural predators so they never run fast enough or for long enough to risk overheating. Reindeer on mainland Europe and America have long been subject to predation from wolves, which chase their prey over long distances. The need to lose excess heat while running fast may curtail the evolution of the short, stocky body form. The value of being able to see predators while the head is lowered for grazing may also account for the longer, narrow face of mainland subspecies compared to Svalbard reindeer.
The insulating properties of furs and feathers can be easily compared by wrapping pelts around heated objects such as bars and measuring their rate of cooling under various conditions. Such observations indicate that in still air, the insulation of all coats of fur or feathers is proportional to their length and thickness, but the texture and secretions from cutaneous glands produce very different properties when exposed to wind and water.
Some small arctic mammals such as lemmings and hamsters spend the winter in burrows and tunnels under the snow, where the air is effectively still all the time. Arctic foxes, and sometimes bears, shelter in snow drifts, and their young are born in dens, but in polar regions there is little shelter from plants, because trees and large shrubs are absent, and there are not many caves or other geological structures formed by flowing water. The ears of arctic foxes are reduced and thickly furred (Figure 2a) and the long, very bushy tail can provide extra insulation to any exposed part of the body when the animal is resting.
The effects of wind are important for large mammals, particularly if, like reindeer, they spend a large proportion of the time foraging in exposed places. In such animals, the outer guard hairs are long and relatively stiff, providing mechanical protection and support for the fine, dense underfur that traps layers of warm air near the skin. Stiff outer feathers and fluffy down combine to insulate birds in much the same way.
In polar homeotherms, fur or feathers often extends over parts of the body that are usually naked in temperate-zone species: the feathers extend along the legs and over the feet of ptarmigan (Figure 5) and snowy owls, and the pawpads of arctic foxes and arctic hares are covered in short, tough fur (Figure 17). The fur of Svalbard reindeer is longer and denser than that of Norwegian reindeer and it covers the ears, eyelids, snout, lips and feet much more extensively.
SAQ 23
Could there be any disadvantages in fur covering all parts of the body?
Answer
The animal's ability to dissipate heat during strenuous exercise or in warm weather is reduced and it risks overheating. When overheated, seals hold their flippers up in the wind or try to get back into water. Reindeer, bears and other terrestrial mammals pant vigorously, but hyperthermia is a real risk, especially for very large or pregnant specimens. The need to dissipate heat during prolonged, strenuous exercise may be one reason why the large hunters, such as wolves, which occur throughout the Russian and Canadian Arctic, are not completely covered in thick fur. In husky dogs (and their wolf ancestors), counter-current blood flow in the legs and nose results in much lower temperatures of the peripheral parts of these organs (Figure 18a), greatly reducing heat loss from them. When the animals are asleep, they tuck their feet and nose into their coat or cover them with the thickly furred tail, but they avoid overheating during long chases by retaining some exposed surfaces through which heat can be lost rapidly. All animals with wettable fur lose heat faster when wet, and most species, including polar bears, shake themselves vigorously immediately after swimming (as dogs do).
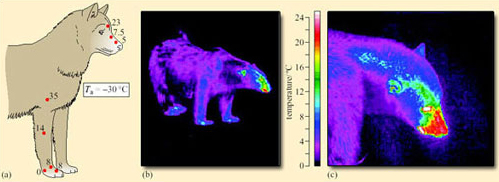
Figures 18b and 18c show similar information obtained by infrared thermography of a free-living polar bear standing on snow in the Canadian Arctic. The nose, mouth, eyes and ears are warm relative to the rest of the body, and are thus the sites of the greatest heat loss per unit area.
SAQ 24
Do the measurements in Figure 18b suggest that this bear was under thermal stress (i.e. too hot or too cold)?
Answer
No. Its feet and lower legs are emitting a moderate amount of heat. Heat lost from the feet would be much curtailed if the animal was too cold, or greatly increased if it was too hot, e.g. after running, as happens in many mammals, including dogs (Figure 18a) and to some extent in ourselves.
SAQ 25
In which of the warm structures revealed by Figure 18c is the high temperature due to (a) rich perfusion with blood or (b) high intrinsic metabolic rate?
Answer
(a) The external ear, eyelids, nose and mouth receive a rich blood supply (even very small wounds bleed profusely), bringing warm blood from the body core to the surface. (b) In contrast, the outer surface of the eye is not perfused with blood (except transiently at the site of injury or infection, when the damaged eye becomes ‘bloodshot’). Its heat is produced locally by the very high metabolic rate of the transparent tissues (lens and cornea) and the numerous tiny neurons that comprise the retina (light-sensitive surface) and the optic nerve.
SAQ 26
Why do bears (and people) keep their eyes almost closed in strong winds and cold weather?
Answer
Figure 18c shows that the surface of the eyeball was above 26° C, the highest surface temperatures recorded from this bear. Even a thin covering such as eyelids would significantly reduce heat loss (as well as protecting the eye from freezing and drying). So arctic animals, especially large species standing out in the wind, view the world through narrow, slit-like eyelids, which confers an aloof, imperious expression.
The surface temperatures of red foxes, kit foxes (another temperate-zone species native to USA) and arctic foxes at air temperatures from −25 to +30° C have also been compared using infrared thermography, producing images such as those shown in Figures 18b and c. There were surprisingly few differences: all foxes lost heat through their legs, paws, ears and snout, but while the two temperate-zone species also lost heat through their thinly furred forehead, this area of the arctic fox is efficiently insulated. In the winter, the forehead of arctic foxes is covered in long, dense fur (Figure 2a), making the animal look like a pet dog, but far from being a trivial character, this tuft of fur is an integral part of the species' adaptation to extreme cold. It is greatly reduced in the greyish-brown summer pelt.
People's breath freezes on beards and eyelashes but ice does not accumulate on the fur of many arctic mammals, notably that of arctic foxes, wolves and wolverines, probably because the microscopic structure of the hair surface and/ or oily secretions from the skin prevent the formation of ice-crystals. Arctic people value such fur, particularly for trimming hoods and mufflers, although it is less soft and silky than mink.
For reasons associated with the erratic food supply (Section 1.2), many arctic mammals are obese, particularly in the winter, a situation that has led to the notion that thick subcutaneous adipose tissue makes an important contribution to insulation. As long ago as the 1950s, measurements on people swimming the English Channel and on men on polar expeditions failed to reveal any firm association between thickness of superficial adipose tissue and the capacity to withstand exposure to cold. Nonetheless, statements that subcutaneous adipose tissue is superficial because it is essential to insulation still appear in many recent textbooks.
SAQ 27
How could you test the hypothesis that subcutaneous adipose tissue is important for thermal insulation?
Answer
The hypothesis predicts that there is normally a thermal gradient across the adipose tissue. In practice, it is quite difficult to measure such a thermal gradient over a long period, and demonstrating it would not prove that adipose tissue (rather than any other superficial tissue such as muscle) is essential to insulation.
Another approach is to compare the partitioning of adipose tissue between internal and superficial depots in arctic and tropical species and look for evidence of selective expansion of the superficial fat in homeothermic animals adapted to cold climates. Polar bears eat seals, and occasionally swim long distances in ice-cold water between hunting grounds. Ice conditions often make their prey inaccessible, forcing them to fast for long periods (Section 3.2), so bears fatten when prey are readily available and are usually obese for large parts of the year. These facts have given rise to the idea that, as in fully marine mammals such as walruses and polar cetaceans (that are often found in the same habitats), adipose tissue makes an important contribution to insulation in polar bears. The data in Figure 19 (Pond and Ramsay, 1992) enable us to test this hypothesis directly. The order Carnivora includes species that share a common ancestry and many habits and range in size from weasels (with a body mass of about 0.1 kg) to bears (with a body mass of up to 700 kg) and are adapted to live in very hot (e.g. Rüppell's fox) and very cold (e.g. arctic fox, polar bear) climates.
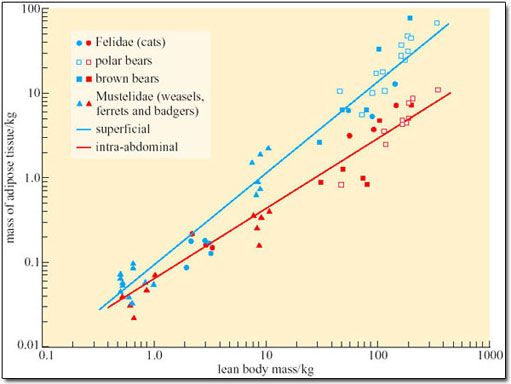
SAQ 28
Is the partitioning of adipose tissue between internal and superficial depots in semi-aquatic arctic polar bears different from that in fully terrestrial temperate-zone carnivores?
Answer
No. The mass of the intra-abdominal depots becomes proportionately smaller and that of the superficial adipose tissue larger with increasing body mass in all species studied. The data for polar bears lie close to the regression lines fitted to the data from the temperate-zone species. There is no evidence for adaptive redistribution of adipose tissue in polar bears. Their skin is warm to the touch and the coarse guard hairs and woolly underfur are probably the principal insulating tissues. The distribution of adipose tissue in mammals native to cold freshwater, such as otters, beavers and muskrats, is also not different from that of related terrestrial species (e.g. badgers, squirrels and lemmings, respectively), providing no evidence that their adipose tissue is adapted to function as an insulator.
As much as 50% of the body mass of large adult bears can be lipid, and selective expansion of the superficial adipose depots relative to the internal depots seems to arise mainly because ‘there is nowhere else for so much fat to go’. The surface area also declines with size in animals of similar shape, so the superficial layer of adipose tissue becomes thicker with increasing body mass, even if it does not become proportionately more massive. There is also proportionately more superficial adipose tissue in large, naturally obese birds: the subcutaneous depots amount to more than 80% of the total adipose tissue in emperor penguins.
4.2 Insulation in aquatic endotherms
Most seals and sealions (order Pinnipedia) are furred. The adult fur usually consists of short, dense stiff guard hairs that are oily from profuse secretions of the sebaceous glands. The hair probably acts like a wet suit of a human diver: a layer of water is trapped around the hair, where it is warmed by body heat and prevents much colder water from coming into direct contact with the skin. Short, oily fur dries quickly when the seals come onto land. Two genera, the northern fur seal (Callorhinus ursinus), in the north Pacific (Figure 20), and eight species of southern fur seals, Arctocephalus spp., in the Pacific and Southern Oceans, have a dense layer of underfur throughout their lives that traps small bubbles of air and keeps the skin dry.

SAQ 29
Are there any disadvantages of such insulation for aquatic mammals?
Answer
During deep dives, the pressure of the water would compress the air bubbles, greatly reducing the insulating efficiency of the fur and increasing the possibility that the skin is wetted. The air also makes the mammal or bird more buoyant, thus hindering diving and swimming underwater.
This kind of insulation is most common in semi-aquatic mammals such as beavers, muskrats and otters, and in the feathers of ducks and penguins, which live mainly in shallow water and spend long periods exposed to very cold air. Newborn seals have a fluffy coat, quite different in both colour and texture from that of the adults, that provides good insulation in air but is ineffective in water. Seal pups cannot survive in very cold water for longer than a few minutes until they moult the neonatal coat and grow the firm, darker adult fur that provides better protection in water. Because the fur is an efficient insulator in air, adult fur seals and the juveniles of many other seal species have long been hunted for their pelts.
In view of the effectiveness of insulating fur among seals, it may seem surprising that one of the most northerly pinnipeds, the walrus (Odobenus rosmarus), and all the whales and dolphins (order Cetacea) are almost hairless, even as neonates. In these marine mammals, the skin and a specialized form of fibrous adipose tissue called blubber are the major insulators. Walrus skin is up to 5 cm thick and the blubber, although minimal or absent over the tail, flippers and parts of the head, is up to 15 cm thick over some areas of the neck and trunk (Figure 21). Measurements on dead tissues indicate that conductance of heat is about half as efficient through adipose tissue as through aqueous tissues such as muscle. However, such passive properties are probably much less important to thermal insulation than counter-current systems of blood vessels, and control of the rate of flow of blood through the tissue, that brings heat from the warm core to the surface. Blood flow through the superficial adipose tissue can be reduced almost to zero for hours without damaging the adipocytes.

SAQ 30
Why cannot the blood flow to muscle be similarly reduced?
Answer
Muscle is much more metabolically active than adipose tissue and cannot remain functional unless supplied with sufficient blood-borne nutrients and oxygen. Deprivation of blood for longer than a few minutes causes permanent damage to most muscles.
When basking out of the water, the skin of walruses is so flushed with blood that it appears pink (Figure 21), particularly in warm weather, which is when they are most often seen by hunters, photographers and biologists. As soon as walruses enter cold water, blood vessels in the skin and outer layers of the blubber constrict, shutting off the circulation almost completely. The animals become dull grey in colour, and much less heat is lost at the surface. During strenuous exercise, or when in warmer water, perfusion can be increased, thereby adjusting accurately the rate of heat loss to internal heat production, as happens in counter-current mechanisms.
SAQ 31
Why is the largest area of pale pink skin in Figure 21 a symmetrical patch between the walrus's eyes? Hint: compare this photograph with Figure 18a and c, and think about where you sweat most when hot!
Answer
The pale patch covers the walrus's forehead, which overlies the forebrain, a metabolically active and functionally important region of the brain that is impaired by even small changes in temperature. Blubber under this area of skin is much thinner than that on the neck and body, perhaps absent altogether. All higher mammals, including dogs (Figure 18a) and bears (Figure 18b and c) lose a lot of heat from this area of the head, protecting the brain from overheating. The human brow sweats profusely and is a good site from which to measure minor changes in overall body temperature, as in fever.
Vigorous exercise is rarely necessary for feeding, because walruses eat mainly bottom-dwelling invertebrates, especially clams and similar burrowing molluscs, which they locate with their sensitive vibrissae (Figure 21), and dig out by squirting jets of water. Being so large (up to 1.5 tonnes), adult walruses have few predators except killer whales and, occasionally, polar bears. Adipose tissue has the advantage of being almost incompressible and, although fat is less dense than water, it contributes less to buoyancy than air trapped in the pelt.
However, restricted blood flow is incompatible with certain other functions of the superficial tissues. Walruses shed the outer layers of the skin each year, possibly as a means of getting rid of external parasites. To support regrowth of the skin, blood perfusion of the superficial tissues is plentiful throughout the moult, and walruses normally spend almost the entire period basking on beaches or ice-floes. Although moulting takes place in mid-summer, walruses can die of cold if forced to spend too much time in the water during this period. Cetaceans spend their entire life in water and moult less efficiently, enabling barnacles and ectoparasites to colonize their skin.
Thick layers of superficial adipose tissue also contribute to insulation in other species of seal, although the relative importance of fur and fat probably differs greatly between the seasons and in different species. The distribution of adipose tissue in most adult seals, dolphins and the small toothed whales suggests that it is adapted to contribute to thermal insulation: the superficial blubber forms an almost continuous layer, albeit of very variable thickness, and adipose tissue is almost absent from inside the abdomen and muscles.
All kinds of seals that have been investigated have surprisingly little superficial adipose tissue at birth, the superficial depots being only 2–4 mm thick in northern fur seal pups that weigh 5–6 kg at birth. Their thick natal coats keep them warm in dry weather on land, but, although their BMR can increase to as much as 18 W kg−1, seal pups quickly become hypothermic if immersed in water or during heavy rain. Furthermore, the distribution of adipose tissue of neonates resembles that of typical terrestrial mammals: as well as several superficial depots, there are significant quantities of adipose tissue inside the abdomen, around the kidneys, and in the pericardium. Some of these internal depots contain adipocytes which appear under the electron microscope to have features in common with BAT: mitochondria are quite numerous but they lack cristae. However, although it may be thermogenic to some extent, the tissue is not true BAT in either structure or metabolism.
Birds replace old, worn feathers with new ones, usually one or twice a year, often just before breeding or migration. Moulting and replacement of the plumage impose heavy demands on the nutrient reserves because large quantities of energy and protein are used in the synthesis of new feathers. Foraging is also difficult or impossible: most large birds cannot fly (in the absence of primary wing feathers) and polar species do not swim because their insulation is so severely impaired that they would become too cold in water. The moult takes 2–5 weeks in emperor penguins and king penguins (Aptenodytes patagonica), during which time they remain on land (or on ice-floes) and fast, losing up to 45% of their body mass and up to 50% of their protein reserves.
4.3 Humans in polar regions
Humans evolved in tropical Africa and gradually colonized colder climates during the Pleistocene ice ages. There have been permanent populations in the Arctic for several thousand years, mostly Inuit (Eskimos) in what are now Canada, Alaska and Greenland, and several groups in northern Europe and Russia, such as the Saami (Lapp) in Scandinavia and the Chukchi in Siberia. Such people do not grow crops and keep only a few domestic animals, mostly for transport (e.g. husky dogs or reindeer), not for food. Until very recently, they lived by hunting seals, walruses, polar bears, fish and wild and semi-domesticated reindeer and, during the brief summer, gathering wild berries.
Adaptation to living in the Arctic has been more technological and cultural than physiological: Inuit (Figure 22) are shorter and stockier than Canadians of European ancestry, but comparisons of the distribution and abundance of their adipose tissue revealed that the native people have less, rather than more, superficial fat.

SAQ 32
What does this comparison suggest about the function of superficial adipose tissue in humans?
Answer
It is not adapted to a role as thermal insulation. Frost damage to exposed parts such as the face and hands is prevented by efficient perfusion with warm blood (see Figure 18), rather than by any form of insulation. Human colonization of the Arctic was made possible by the effective use of animal skins as clothing.
Adaptations of digestion and metabolism have evolved among Inuit: although their diet was very rich in fat and protein, and for 9 months of the year included almost no fruit or vegetables, diseases such as obesity, diabetes, scurvy, rickets, dental caries, constipation and colon cancer were rare. However, obesity, diabetes and dental caries have become much more common during the last 40–50 years since they adopted a western diet. Inuit have never grown or stored crops, and so alcoholic drinks produced from fermented carbohydrates (i.e. grain, potatoes, fruit or sugar) were never part of their diet: alcohol dehydrogenase, the enzyme that detoxifies alcohol, is present in very small quantities in their livers and it is not as readily induced as it is in people whose ancestors have a long tradition of drinking alcoholic beverages. Consequently, grown men are easily intoxicated by as little as 0.25 l (half a pint) of beer.
The capacity of the human nose to conserve moisture by warming and hydrating inhaled air and reclaiming the heat and moisture of exhaled air (shown in the dog and bear in Figure 18), is much less efficient than the long nasal turbinals of native arctic mammals such as bears, reindeer and wolves. The ability to breathe steadily through the nose rather than through the mouth improves with practice, but most inexperienced visitors to polar regions are bothered as much by thirst as by cold.
Living in such a severe climate is very tough: archaeological studies suggest that human habitation of arctic regions was often transient, with many settlements being abandoned when the climate worsened or food became scarce. Until very recently, resources were never abundant enough to support the development of large, dense cities or towns.
People from the temperate zone have only recently explored the high arctic regions, attracted by opportunities for hunting fur-bearing animals (seals, bears, beaver, lynx, wolves, arctic fox, musk rat, otters), and whales and other marine mammals for their meat and oil, and the search for gold, crude oil and other minerals. European expeditions, such those led by the Dutch sea captain, Willem Barents, in 1596–1597 and by the Russian-financed German explorer, Vitus Bering, in 1741, visited the Arctic Ocean and many of its islands, including the Svalbard Archipelago (see Section 1.1). Sir John Franklin led several British expeditions to northern Canada and the islands off the north coast, starting in 1819. Although equipped with the latest ships and extensive provisions, and assisted by the Inuit, Franklin and almost all his crew died on the final journey. Their primary objective, to find and map the North-West Passage from Europe to Asia, was never realized. No permanent settlements of Europeans were established in the high Arctic until the 20th century.
There is no pre-historic evidence for humans on Antarctica. The voyage of Captain James Cook in 1772–1773 is the first known exploration of the Southern Ocean. Fisherman and hunters of whales and seals landed on many of the islands during the 18th and 19th centuries, but Antarctica itself was not explored until the first decade of the 20th century. Although research in and around Antarctica has been much expanded since the 1960s, there is still no permanent, breeding human population.
4.3.1 Summary of Section 4
Many polar mammals and birds are obese because their food supply is highly seasonal or erratic. In large species, proportionately more adipose tissue accumulates in the superficial depots and less in the internal depots in tropical, temperate-zone and polar animals. There is evidence for redistribution of adipose tissue as an adaptation to thermal insulation only in pinnipeds and smaller cetaceans. Unlike fur, adipose tissue is incompressible: its effectiveness as insulation depends upon rapid, efficient control of blood perfusion through it and the skin. Humans are basically tropical and have colonized arctic regions only very recently in evolutionary terms, so they have minimal anatomical and physiological adaptations to the environment and are capable of only limited acclimatization.
5 Polar ectotherms
5.1 Introduction
The land and shallow water experience at least a brief summer at high latitudes, so terrestrial and freshwater ectotherms can be active during warm periods and hibernate when the temperature is below freezing. In contrast, the polar seas are not warmed significantly by the Sun: the mass of water is too large and sea-ice covered in snow reflects sunlight very well. The seawater beneath the permanent sea-ice is continuously at between −1.9 and +6° C, so its inhabitants complete their entire life cycle at temperatures at which tropical ectotherms would die and most temperate-zone species would become torpid. There is much less mixing between warm and cold currents around Antarctica, so in the southern oceans, temperature zones are sharply delimited and have distinctive faunas. However, movements of water currents in the North Atlantic and North Pacific cause quite large seasonal changes in water temperature around the Arctic and hence less clearly defined faunal zones.
The common arctic fish are closely related to species in north temperate-zone waters. They include two salmonids, the capelin (Mallotus villosus) and the arctic char (Salvelinus alpinus), which breeds in rivers but spends part of its adult life in the sea, sculpins (family Cottidae), various flatfish such as polar halibut (Reinhardtius hippo glossoides) and flounder (Pleuronectes americanus) and members of the cod family, such as arctic cod (Arctogadus glacialis) and haddock. Many of the most abundant and widespread fish around Antarctica belong to a suborder Notothenioides of the order Perciformes (perches). Nototheniids (Figure 23) probably evolved in the oceans around Antarctica during the last 20–30 million years, and the living species are almost confined to that region. In contrast to the Arctic Ocean, there are very few species of the cod (Gadiformes), herring (Clupeiformes) and salmon (Salmoniformes) families in the Southern Ocean. Only a few chondrichthyan fish live in polar waters, among them the Greenland shark (Somniosus microcephalus).
5.2 Passive properties
Freezing is nearly always harmful to living cells because the tertiary structure of hydrophilic molecules such as proteins is disrupted and the permeability of membranes is drastically altered. The concentration of solutes in the blood of teleost fish is only about half that of seawater so, while seawater freezes at −1.9° C, fish blood would be expected to freeze at −1 to −0.6° C. One way in which fish living in very cold seawater avoid what seems like inevitable disaster is by supercooling: the body fluids can remain indefinitely below −0.6° C, provided they do not come into contact with any ice-crystals. The consequences of so doing were first demonstrated over 50 years ago by the Norwegian physiologist, Per Scholander (Figure 24).
SAQ 33
What tissues are the likely route for entry of ice-crystals into the fish?
Answer
The gills, which present no effective barrier to ice-crystals, the gut when food is ingested, and the flow of urine from the excretory system.
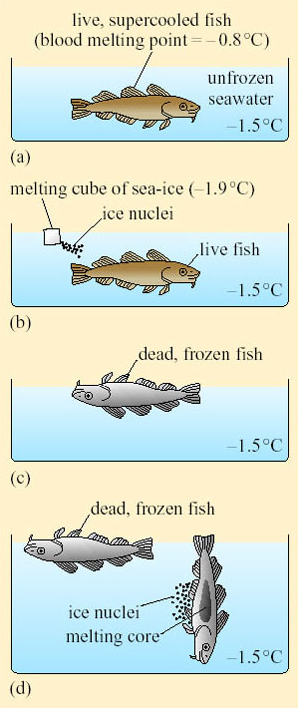
Except during urination, the urethra is closed tightly by a muscular sphincter lined with large quantities of mucus, thereby minimizing the risk of ice-crystals forming in the relatively dilute urine. Ice floats in water and the deeper layers of the oceans are usually slightly warmer, −1.8° C, than the surface water. So one way of avoiding the fate described on Figure 24 is to remain in deeper water. However, some fish, notably the capelin, spawn on beaches where the air temperature can be much colder than that of the sea. The sticky secretions on the outside of the eggs both stick them to the rocks and promote supercooling down to −5° C, but if the shells are pierced, tiny ice-crystals quickly form and the embryos freeze at −1.4° C.
Freezing is also avoided by the presence of ‘antifreezes’ called cryoprotectants (κρυoσ, kryos or κρυμoσ, krymos=frost) in the blood. Natural cryoprotectants are usually glycopeptides or peptides of molecular weight 2400–36000 that bind to ice-crystals and prevent them from growing larger than tiny nuclei. These cryoprotectants are present in almost all body fluids, including the blood, the cerebrospinal fluid, the peritoneal fluid, the interstitial fluid of the muscles, and (via bile secretions) the lumen of the gut. Other body fluids, such as the ocular fluid in the eye, are protected from contact with ice-crystals by the surrounding tissues. Cryoprotectant molecules are not eliminated by the kidney because most polar fish that have them also have aglomerular kidneys: their urine is formed by secretion into the nephron rather than by filtration.
At least eight different cryoprotectant molecules have already been identified, and almost all antarctic fish have some kind of antifreeze in their body fluids, usually throughout the year. Similar cryoprotectants have evolved in several kinds of arctic fish, but such adaptations are less widespread and cryoprotectants are present only during the winter in many species. In the northern oceans, most of the fish (and their mammalian and avian predators stay in the highly productive waters at the edge of the iceshelf and avoid direct contact with frozen seawater. Other cryoprotectant agents have evolved in terrestrial arthropods, amphibians and reptiles that hibernate in sub-arctic areas of Canada and Russia.
5.2.1 Blood pigments
The solubility of oxygen (and of many other gases) in water increases with decreasing temperature: at 0° C, seawater holds 1.6 times as much oxygen when saturated as at 20° C. This fact, and continual disturbance by frequent storms, mean that the surface waters of polar oceans are very well oxygenated. A family of 17 species of nototheniid fish, the Channichthyidae, have no erythrocytes, no haemoglobin and almost no myoglobin at all stages of the life cycle (Section 1.5).
SAQ 34
What would such fish look like?
Answer
Unless the skin is pigmented, they would be almost colourless. Collagenous tissues usually scatter light evenly, thus appearing dull white, like mammalian fascia or tendon.
SAQ 35
For what tissue is being coloured essential to its function?
Answer
Visual pigments called opsins absorb light in the retina, making the back of the eye appear black. Fish adapted to function in dim light have relative large eyes. Sea-ice transmits little sunlight, and daylength at high latitudes is often very short (see Figure 1). Many fish in the Southern Ocean around Antarctica (Figure 23) have large eyes in which the dense, dark retinal pigments stand out against the pale bodies, making them conspicuous. Pigment cells in the skin provide camouflage for many species. Trematomus bernacchii (Figure 23c) is mottled brown, black and pink when it rests on rocks in shallow water, but its pigment cells contract when it is deep water, making the skin uniformly pale. Its common name, emerald rockcod, refers to several green spots on its broad, flexible pectoral fins. Pleuragramma antarcticum (Figure 23a) is called the silver fish because its fine scales reflect light, camouflaging its outline as it moves.
The most thoroughly studied species, the icefish (Chaenocephalus aceratus, Figure 23e), is almost transparent because the proteins in its skin and muscles are sufficiently ordered to enable light to pass through them with minimal scattering.
SAQ 36
How could the absence of erythrocytes improve blood flow?
Answer
The viscosity of most fluids, including water, increases with decreasing temperature, so the heart must pump harder to maintain the circulation of the blood. Blood cells, many of which are quite large relative to the diameter of the vessels through which they pass, make a major contribution to the viscosity, so the elimination of erythrocytes would make the blood much less viscous and hence reduce the work of pumping.
As well as being large (up to 1m long), icefish are active predators that swim in the surface waters and use oxygen at about the same rate as red-blooded antarctic fish such as Pleuragramma or Notothenia (see Figures 23a and b). Icefish blood contains solutes and a few white blood cells, so it is a yellowish, watery fluid similar to mammalian lymph. Large volumes of it flow in wide capillaries propelled by a heart that pumps out three to four times as much blood as that of a red-blooded fish of similar size and habits. The bulbus arteriosus on the anterior side of the heart is greatly expanded and is the only muscular tissue to contain any myoglobin. Measurements on the uptake and circulation of oxygen in captive icefish suggest that, as well as the gills, the thin, scaleless skin is important as a site of gas exchange, with up to 8% of the oxygen absorbed through the tail skin alone. At low temperatures, the blood of icefish circulates faster and takes up nearly as much oxygen from the water as red blood, although transfer of oxygen to tissues is less efficient. However, in even slightly warmer water, these advantages disappear and icefish suffocate.
SAQ 37
What other aspect of energy metabolism is likely to be different in icefish?
Answer
Anaerobic metabolism. Without myoglobin, their muscles are unable to store oxygen so the fish quickly become anoxic during very fast swimming or in deoxygenated water. They tire quickly after a brief burst of swimming and cannot tolerate the build up of more than a very little lactic acid in the muscles and, of course, they do not survive in warmer, less oxygenated water.
5.3 Metabolism
Molecules diffuse more slowly at low temperature: measurements of the rates of diffusion of small molecules such as lactic acid, Ca2+ and analogues of glucose and ATP through fish muscles produced Q10 values of 1.75–2.04 between 5 and 25° C. Nearly all enzyme reactions are slower at low temperatures (although sometimes whole pathways can be faster if an inhibitor is more inhibited by low temperature than are the catalysts). So, in the absence of temperature compensation, most metabolic processes, including contraction and relaxation of muscle, digestion and growth, are slowed. The Q10 values of most enzyme-mediated processes that have been studied directly are in the range of 1.5–3.0.
One of the fundamental cellular processes that has been most intensively studied is the maintenance of ion gradients across the cell membrane. This system is particularly relevant to cold adaptation because it is an almost universal property of cells and because ions leak into the cell passively but are actively extruded by an ATP-based pump. Inward movement of ions through ion channels is basically a physical process, for which the Q10 is about 1.2–1.4 in the range 0–10° C, but, like other active, enzymatic mechanisms, the Q10 of ATP-producing pathways is 2–3. So as the temperature falls, extrusion cannot keep up with inflow, ions accumulate in the cells, and the membrane potential falls, with disastrous effects in neurons, muscle, kidney and many other kinds of cells. In theory, stable coupling between the two processes at low temperatures could be achieved either by increasing the capacity for active transport of ions or by decreasing membrane permeability.
SAQ 38
What would be the implications of these adaptations for BMR and exercise habits?
Answer
ATP production and utilization are major components of BMR, so more active extrusion of ions would lead to higher BMR. Decreasing membrane permeability would reduce the need for ion pumping, leading to lower BMR and lower oxygen utilization, but also to diminished capacity for osmoregulation and sluggish movement.
Polar fish have recently been studied intensively both in the wild and in the laboratory. The resting metabolic rate of several antarctic fish at −2° C proved to be at least twice as high as that expected from extrapolation of BMR data of temperate-zone or tropical fish to this temperature. However, if such warm-water fish were cooled to this temperature, they would probably not be able to swim at all and would quickly die, so the comparison is not really valid. A more relevant comparison is with temperate-zone fish that live in the deep sea, where the water temperature is always 0–4° C. When such species are compared at their normal physiological temperatures of about 0° C, the few antarctic fish that have been studied are found to have relatively high BMR. Their respiratory capacity is also more efficient: at 0° C, the isolated gills of the emerald rockcod (Figure 23c) take up oxygen at the same rate as those of the common goldfish at 15° C.
5.3.1 Muscles
The rates of muscle contraction and relaxation, and the maximum force generated, are complex enzymatic processes that determine speed of swimming. Ian Johnston of St Andrews University (Johnston, 1989) has compared the maximum tension of muscle fibres isolated from several species of antarctic, temperate-zone and tropical fish (Figure 25).
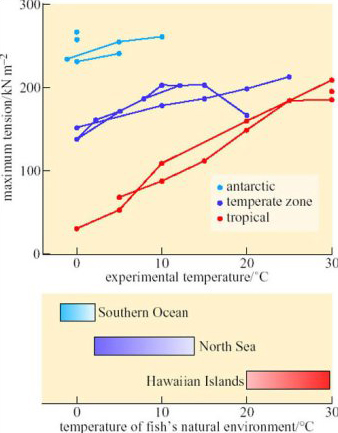
SAQ 39
Do the data on Figure 25 provide evidence for temperature compensation of the contractile mechanism?
Answer
Yes. Between 0 and 10° C, muscles from antarctic fish generate forces five to ten times larger than those measured from muscles of tropical species.
However, these properties of isolated muscle fibres did not match well with studies of individual molecules and whole animals. Temperature compensation could not be demonstrated in the maximum activity of some key enzymes in muscle contraction (e.g. ATPase) and many mitochondrial enzymes (e.g. cytochrome oxidases) studied in vitro. When temperate-zone fish such as goldfish (Carassius auratus), eels (Anguilla anguilla) and carp (Carassius carassius) are acclimated to low temperatures, the proportion of the volume of red muscle fibres occupied by mitochondria increases from 14% at 28° C to 25% at 2° C, indicating that oxidative capacity is maintained by the presence of more mitochondria, rather than by temperature compensation of the enzymes. Antarctic fish also generally have more and/or larger mitochondria but the data are not very clear cut: the proportion of the volume of red muscle fibres occupied by mitochondria ranges from 13–56% in the five antarctic species studied, compared to 4–45% in temperate-zone fish. The muscle fibres of the icefish (Chaenocephalus aceratus, Figure 26a) are more than half mitochondria, leaving little room for the contractile mechanism itself. Those of red-blooded antarctic fish, such as Notothenia gibberifrons (Figure 26b), contain a greater proportion of contractile myofibrils than the icefish, but mitochondria are still more abundant than in temperate-zone fish, particularly towards the edges of the muscle fibres near the blood vessel.
SAQ 40
How could mitochondria arranged as in Figure 26 adapt the muscles to activity at low temperature?
Answer
Diffusion is slower in the cold. Delays in metabolites such as ATP reaching the contractile proteins are minimized if numerous mitochondria are interspersed between the muscle fibres, thereby shortening the mean distance between the mitochondria and the ATP-using enzymes.
Biologists from the University of Maine compared the maximum activities at 1° C of several enzymes in the swimming muscles and the heart of two antarctic fish, Notothenia gibberifrons (similar to Figure 23b) and Trematomus newnesi (similar to Figure 23c), with those of two species of similar size and habits caught in the western Atlantic Ocean off the coast of Delaware, USA. They found that the activities of enzymes involved in lipid catabolism and aerobic respiration, such as carnitine palmitoyltransferase and 3-hydroxyacyl CoA dehydrogenase, were 1.3–27.0 times higher in the red muscles of the antarctic species than in those of the temperate-zone species and the Q10 values were less than 2. However, the activities of phosphofructokinase, pyruvate kinase and lactate dehydrogenase that are essential to anaerobic utilization of carbohydrates were either not significantly different or were lower in the polar species. Thus these antarctic fish seem to be equipped to use lipid fuels more efficiently than carbohydrate fuels.
Maximum swimming speed during brief ‘bursts’ of activity (e.g. when escaping from a predator) has been measured accurately in only two species of antarctic fish, and was found to be at the lower end of the range found in temperate-zone fish of similar size and body shape. Clearly, the situation is complicated and further research on a greater range of species is necessary to understand adaptation to low temperatures. Nonetheless, even such limited information enables us to identify some principles of adaptation to polar conditions in ectotherms.
SAQ 41
With which of the two mechanisms for maintaining ionic balance at low temperature are these data most consistent?
Answer
Taken together, the observations suggest that antarctic fish living in surface waters achieve temperature compensation by increased activity of the ionic pump.
SAQ 42
Why would this mechanism be better for antarctic fish living in surface waters?
Answer
Slow movement is unlikely to be adaptive where fast-swimming, endothermic predators such as penguins and seals are about. There are relatively few surface-swimming fish in antarctic waters. Most fish live on or near the bottom, or in deep waters, out of reach of most air-breathing predators.
The silver fish (Pleuragramma antarcticum; Figure 23a) lives in the surface layers of coastal waters and eats pelagic invertebrates. Around McMurdo Sound in Antarctica, silver fish are known to be an important food for penguins, skuas and Weddell seals (Leptonychotes weddellii). Although its high density of mitochondria must increase its BMR and the energy cost of swimming, such adaptations to quick responses and fast escape are probably essential to avoiding predation. In contrast, most deep-sea fishes that have been investigated (and only a few species have been kept alive in surface laboratories for long enough to be studied) have lower BMR than expected and probably compensate for low temperature by reducing membrane permeability. Mammalian and avian predators are absent in the deep sea, and food (and probably also oxygen) is scarce, so the alternative mechanism for maintaining the potential gradient across the cell membranes is more efficient.
Another peculiar and consistent feature of nototheniid fish is that most species, including P. antarcticum (Figure 23a), Dissostichus mawsoni (Figure 23d) and species of Notothenia and Trematomus, have numerous sacs of lipid within and around their swimming muscles and between the muscles and the skin. Several functions have been suggested: energy stores, buoyancy (which could be important in fish that lack swim bladders) and more recently, oxygen diffusion. The solubility of oxygen in lipid is four times higher than in aqueous cytoplasm, and, particularly in fish in which blood pigments are reduced or absent, lipids closely associated with muscles may facilitate oxygenation of the tissues.
You may have noticed that in Figure 25 the contraction of muscles from tropical and temperate-zone fish were measured over a temperature range from 0° C to 25–30° C, but there are no data for the muscles of antarctic fish above 10° C. Many polar fish tolerate only a very narrow range of temperatures and die within minutes if warmed more than a few degrees above the temperature of the water in which they normally live. Exactly why they die is not clear, but a likely cause is widely different Q10 values of enzymes in critical metabolic pathways, such as ion pumps or mitochondria: a small change of temperature puts the whole pathway ‘out of kilter’, causing metabolic intermediates to accumulate to toxic concentrations. In this respect, polar fish resemble non-hibernating homeotherms such as humans and rats: their metabolism is seriously, often irreversibly, disrupted by small departures from their normal body temperature. This property, of course, makes it much more difficult to transport such fish alive and to keep them in captivity for long enough to study habits such as breeding, growth and dietary preferences.
5.4 Fatty acids as indicators of diet
Although polar fish and invertebrates are difficult to study alive for the reasons just described, some information about their diet and habits can be obtained from analysis of the lipid composition of their tissues. At high latitudes, the supply of most kinds of marine food changes with the seasons, just as it does on land, and many fish eat little or nothing for long periods, living off their reserves of triacylglycerols. Lipids are major fuels for polar fish and, although many fish have little or no adipose tissue, storage lipids are deposited in the muscles or liver, sometimes in large quantities.
The vertebrate digestive system breaks down proteins, carbohydrates and most other nutrients to amino acids, glucose or other small molecules containing just a few carbon atoms. In contrast, triacylglycerols and phospholipids from membranes are hydrolysed to glycerol and fatty acids, but in simple-stomached animals, the latter are not further broken down: medium- and long-chain fatty acids pass intact into the blood and from there into the tissues.
SAQ 43
Name three different roles that long-chain fatty acids could play in vertebrate tissues.
Answer
The fatty acids may be oxidized for ATP production, or incorporated into the cell fabric, primarily as membrane phospholipids, but also combined with other molecules to form acylated proteins or glycolipids, or re-esterified into storage triacylglycerols. Certain polyunsaturated fatty acids could also become signal molecules.
In carnivores and other simple-stomached animals, almost all the fatty acids deposited in membrane phospholipids and storage triacylglycerols are unaltered, so the fatty acid composition of such components of animal tissue reflects that of the diet over the previous weeks or months. The relationship between diet and tissue composition is different in ruminants such as reindeer: microbes in the rumen convert much of the dietary carbohydrates to short-chain fatty acids, and alter the structure of the plant lipids. The reindeer's liver and adipocytes also synthesize long-chain fatty acids from short-chain fatty acids.
Hundreds of different fatty acids are known from plants, microbes and animals, but many are chemically so similar that until recently, separating and identifying them was slow and difficult. Over the past few decades, instruments such gas-liquid chromatographs have become both more accurate and easier to use, enabling biologists to identify and quantify dozens of different fatty acids in small samples of tissue. Such ‘fatty acid profiles’ are particularly valuable for understanding the diets of cold-water fish such as herring, sprats, capelin, mackerel and halibut, which usually store more lipid than their tropical relatives. In the North Atlantic, these commercially important fish often fluctuate greatly in numbers, and in their flavour and nutritional quality as human food. The causes of changes in their abundance are poorly understood, but may be related to fluctuations in the availability of their prey arising from instability of weather, and movements of sea-ice.
Marine mammals including most kinds of seals and many whales and porpoises also eat fish, usually swallowing their prey whole, and digesting it completely. Most of the prey species contain some storage lipids, which pass almost unmodified into the predators' tissue. The availability of the different kinds of prey species is also major cause of their breeding success. Dietary analysis also enables scientists to monitor the recovery of populations of species after legislation restricting commercial hunting is enforced.
SAQ 44
What anatomical features of these marine mammals would facilitate using fatty acid profiles to obtain information about recent diet?
Answer
The adipose tissue is plentiful and forms superficial blubber that usually receives little blood perfusion. Biopsies of up to a gram of blubber can be taken with minimal injury to the animal. This quantity of tissue yields sufficient triacylglycerols that can be analysed to provide information about what the mammals have been eating. However, the outer layers of blubber, that are cool most of the time, contain more polyunsaturated fatty acids than the inner, warmer layers. Lower perfusion with blood probably also means that triacylglycerols are mobilized and deposited more slowly in the outer layers. To avoid sampling errors, blubber biopsies must always be taken from the same relative depth of blubber (not easy if the total thickness changes with anatomical site and with whole-body fattening).
The conclusions are often surprising, and have revised our understanding of what some kinds of seals and whales are finding to eat, especially in polar regions. Populations of the same species in different areas prove to have quite different diets, indicating that predators must be behaviourally, anatomically and metabolically highly adaptable.
5.4.1 Summary of Section 5
Several anatomical and biochemical adaptations to living in very cold water have evolved in polar fish, particularly those of the southern oceans, which have evolved in isolation for many millions of years. Cold, turbulent water is rich in oxygen. One family of fairly large fish lacks blood pigments but its blood is less viscous and it has additional respiratory surfaces. Many fish have cryoprotectants in the blood and other body fluids, and the muscles of some contain numerous mitochondria and are adapted to use lipid as fuel in preference to carbohydrate. Many polar fish tolerate only a narrow range of temperatures and quickly die if exposed to water even slightly warmer than that in which they normally live. The fatty acid composition of the tissue (the ‘fatty acid profile’) provides information about the animals' recent diet, which varies greatly with season and location.
6 Conclusion
There is much more to living in polar regions than insulation against the cold. Food may be very scattered both in space and in time and breeding must be tightly synchronized to seasons and food availability. Some of the most spectacular examples of natural obesity and efficient regulation of appetite are found among polar animals. The study of such species not only demonstrates that it is possible to remain healthy and active when very obese and during prolonged fasting, it also helps us to identify the similarities and differences between natural and pathological or artificial obesity. Until recently, people were unable to remain in the Arctic and Antarctic for long enough to study the fauna and flora in detail, but as new techniques become available we can expect to find out more about these organisms living at the extremes of climate.
Endothermic birds and mammals comprise a large part of the polar faunas and survive mainly by more efficient insulation and energy budgeting. Profound modifications of the circulation and muscles enable them to avoid excessive predation from marine mammals and birds, whose body temperature may be 40° C warmer. Some teleost fish and numerous invertebrates (including crustaceans, molluscs and several phyla of worms) have also evolved ways of completing their entire life cycle in the cold. Reptiles and amphibians have failed to adapt to continuous cold and are absent from the arctic and antarctic fauna, although a few species occur in cold areas at lower latitudes, where they hibernate during the winter and feed and reproduce during the brief warm summer.
6.1 Extra-terrestrial life?
Polar biology not only enables us to understand an extensive but, until recently little explored habitat, it may also be an important guide to life elsewhere in the universe. Within the Solar System, extra-terrestrial life is now thought to be most likely on the planet Mars, and on Ganymede and Europa, the largest and smallest of the major moons of Jupiter (the Italian physicist and astronomer Galileo (1564–1642) described and named the four largest moons orbiting Jupiter in 1609–1610; by 2003, at least 61 satellites of Jupiter had been identified). This hypothesis is based mainly upon analysis of the close-up pictures and physical measurements sent back by space probes from the mid−1970s onwards. Mars and Ganymede probably have ice-covered poles, beneath which may be water; the extensive ice-covered seas of Europa may also contain large quantities of water, kept warm by volcanic activity in the rocks below. All these habits resemble the polar regions on Earth: ice, probably cracked and perforated, covers water that is only dimly illuminated by sunlight.
Many astronomers believe that extra-terrestrial organisms would probably resemble the psychrophiles found in the Arctic and Antarctic. Terrestrial psychrophiles include some eukaryotes (mostly fungi and protoctists), but the majority are bacteria, including a wide variety of the primitive Archaebacteria, believed to be the earliest kind of life on Earth. Their metabolism is distinctive in various fundamental ways, including unusual features of their genetic code and cell membranes. The astronomers' current interest in detecting traces of contemporary or extinct extra-terrestrial life is stimulating much new research into the structure, physiology and evolution of psychrophiles.
Course questions
Question 1
Which of the factors (a)-(g) is/are a valid reason(s) for the fact that penguins are numerous and diverse around Antarctica but absent from the Arctic?
(a) The climate in the Arctic is too severe for penguins.
(b) There are not enough fish in Arctic waters to sustain a population of penguins.
(c) Penguins are excluded from the Arctic by the presence of bears.
(d) Penguins are excluded from the Arctic by the presence of seals.
(e) Penguins evolved in the Southern Hemisphere and have never occurred naturally in or around Europe, Asia or North America.
(f) In general, there are fewer birds in the Arctic than in the Antarctic.
(g) In general, there are fewer vertebrate animals in the Arctic than in the Antarctic.
Answer
Only (e) is correct. In general, the climate is harsher in Antarctica than in the Arctic. There are plenty of fish in the Arctic Ocean (although they are of different species from those in the Southern Ocean). Polar bears eat seals, not birds although they might eat a penguin if the species ever met). Leopard seals are common in coastal waters of the Southern Ocean and penguins are their principal prey, but the two species have coexisted successfully for millions of years. Many migratory birds breed in the Arctic and a few, notably ptarmigan, are resident. With bears, reindeer, arctic foxes, birds, walruses and numerous fish, as well as seals, the Arctic has more species of vertebrates than Antarctica, which is without any fully terrestrial vertebrates.
Question 2
What is the evidence that endogenous factors as well as daylength control food intake and breeding? Why are such dual control mechanisms adaptive for polar animals?
Answer
Svalbard and Norwegian reindeer ate different amounts even when they had unlimited access to food and were exposed to the same environmental conditions, so the appetites of the two subspecies must be at least partially under endogenous control (see Figure 3).
The effects of exposure to continuous light on food intake and body mass depends upon the season at which the treatment is started. At very high latitudes, there is continuous light or continuous darkness for several months (see Figure 1), so daylength would not, by itself, be a sufficiently precise clue to seasonal breeding that must be accurately timed to favourable weather and food supply, e.g. for ptarmigan (see Figure 6).
Question 3
Which of the statements (a)–(g) about the food intake and metabolism of polar mammals and birds is/are generally true?
There are many more animals relative to the food supply in polar regions than in the tropics.
The food supply in polar regions is highly seasonal and/or irregular.
Being obese makes animals lethargic.
Very lean animals are incapable of strenuous physical activity.
Polar animals have fewer predators than tropical animals so they can afford to be fat and lazy.
The only food available in polar regions is less nutritious than that available in the tropics.
Polar animals have a higher metabolic rate and so need more food than those in the tropics.
Answer
Only (b) is generally true. It would be impossible to sustain permanently more animals than the food supply could support. At the beginning of fasting, bears are both fat and lethargic, but one does not cause the other: male penguins are least active during incubation, and walk briskly back to the open sea at the end of the fast when they are thin. Bears and penguins, like large animals everywhere, have few predators, but smaller birds, lemmings and fish have plenty of predators. Since many fish and terrestrial animals are fat while in arctic regions, it cannot be true that the available food is less nutritious. There is no clear evidence for (g): BMR actually falls below normal for weeks at a time in bears and penguins and is less in winter- than summer-acclimatized foxes (Figure 4).
Question 4
Explain in a few sentences why measurements of the concentration of ICTP and PICP in blood samples taken during peak activity and in the middle of the dormant period failed to explain how black bears avoided increased risk of bone fracture at emergence from dormancy.
Answer
The marker for bone breakdown, ICTP, was more abundant during dormancy, but the differences between denning and activity for the same bears were not significant for PICP, the marker for bone formation (Figure 13). These observations explain how the skeleton is weakened during dormancy, but not how it is reformed during activity. PICP has a brief peak at the start of the active period (Figure 14), probably indicating rapid strengthening of the skeleton that was not detected by this sampling procedure.
Question 5
What information relevant to the metabolism of energy and/or proteins can be obtained from measurements of:
body temperature
RER
the composition of the blood
Answer
(a) Body temperature is a crude and somewhat indirect measure of activity. In both bears and penguins, body temperature falls slightly at the start of fasting (see Figure 11). Since there is no evidence that the body insulation has changed, the rate of production of heat (i.e. BMR) must have decreased. Body temperature rises abruptly during egg-laying in female penguins; the synthesis of components of the relatively large egg and the effort of laying would raise BMR.
(b) RER provides information about the chemical composition of the fuel broken down by respiration. An RER close to 1 indicates that carbohydrates are being used; a lower RER means that proteins and/or lipids are being used.
(c) Chemical analysis of blood plasma provides detailed information about the concentrations of different fuels available to cells (e.g. fatty acids, glucose, T-hydroxybutyrate) and about the rates of formation and accumulation of breakdown products of metabolism (e.g. urea, creatinine, T-hydroxybutyrate).
Question 6
Which of the statements (a)-(h) about the structure and arrangement of adipose tissue is/are true?
All polar mammals have thick subcutaneous adipose tissue.
Birds, whether polar or not, do not have thick subcutaneous adipose tissue.
Polar bears have thick subcutaneous adipose tissue because they live in very cold climates and swim in the sea.
In Carnivora, the partitioning of adipose tissue between superficial and intra- abdominal depots depends upon body size.
In Carnivora, the partitioning of adipose tissue between superficial and intra- abdominal depots depends upon habits and habitat.
In naturally obese mammals, fatter individuals always have more adipocytes than thinner individuals.
In naturally obese mammals, as an individual gets fatter, its adipocytes enlarge.
The number and size of adipocytes in relation to fatness are quite variable in naturally obese mammals and in humans.
Answer
Only (d), (g) and (h) are true. Many arctic mammals have subcutaneous adipose tissue for part of the year, because many are large and they naturally become obese because the food supply is seasonal. However, in mammals, having subcutaneous adipose tissue is not an essential or unique feature of living in an arctic climate. In Carnivora, including bears, the partitioning of adipose tissue is related to body size, not to habits or habitat (see Figure 16). In any one animal, the adipocytes enlarge with increasing fatness, but the total adipocyte complement, and hence the relationship between mean adipocyte size and fatness, differs from individual to individual.
Question 7
Explain in a few sentences:
The effects of the absence of red blood cells on delivery of oxygen to the muscles of Chaenocephalus.
Why most fish living in very cold water remain on or very near the bottom, but there are plenty of invertebrates in mid- and surface waters.
Answer
(a) Without haemoglobin in red blood cells, oxygen is carried in solution in the blood, but the solubility of oxygen in water increases with decreasing temperature. The enormous reduction in density of cells in the blood offsets the increase in its viscosity at low temperature, so the blood flows faster for the same work of pumping by the heart. These factors mean that at −1.5 °C, the muscles of an icefish receive nearly as much oxygen as those of a fish with haemoglobin, but such fish would be unable to swim efficiently in warm water.
(b) The salt concentration of the body fluids of fish is less than that of seawater so their supercooled body fluids are at risk from freezing if they come into contact with ice crystals (Figure 24). Because ice is less dense than water, it floats at the surface. The salt concentration of the body fluids of most invertebrates is similar to that of seawater, so they are not vulnerable to freezing until the seawater itself freezes. There are also more diving birds such as penguins and skuas, and marine mammals, all of which eat fish, in surface waters.
Question 8
Explain in a few sentences why:
The diet of large fish and marine mammals changes seasonally, and may differ greatly for members of the same species found at different sites.
The relative abundance of the fatty acids in adipose tissue triacylglycerols of herbivores such as reindeer would reveal little about the diets of animals found in different areas.
Answer
(a) The abundance of marine plankton, and hence of all the animals that eat it, changes greatly with the season, and with currents and ice movements. The climate, and hence the presence and availability of prey species, change erratically and may differ greatly between apparently similar sites. So large predatory fish and mammals (and seabirds such as albatross) range over a wide area and have a varied diet.
(b) The composition of fatty acids in the storage lipids is determined by the metabolism of the microbes in the rumen and by that of the reindeer tissues, more than by the composition of fatty acids in the diet.
References
Acknowledgements
The content acknowledged below is Proprietary (see terms and conditions) and is used under licence.
Grateful acknowledgement is made to the following sources for permission to reproduce material in this course:
Course image: International Fund for Animal Welfare in Flickr made available under Creative Commons Attribution-NonCommercial 2.0 Licence.
Figures 2a, 5 Dr Alison Ames;
Figures 2b, 17, 18b, c, 22a and b Caroline Pond, Open University;
Figure 7 Mark Eaton;
Figure 10a Bryan and Cherry Alexander Photography;
Figure 10b © Kim Crosbie;
Figures 13 and 14 Donahue, S. W. et al. (2003) Serum markers of bone…, Clinical Orthopaedics and Related Research, 406. Copyright © Dr S. W. Donahue;
Figure 18a Dill, P. B. and Irving, L. (1964) Polar biology, Handbook of Physiology, Chapter 5, American Physiological Society;
Figure 18b, c Dr Malcolm A. Ramsay, University of Saskatchewan, Canada;
Figure 20 Captain Budd Christman, Office of NOAA Photo Library;
Figure 21 Office of NOAA Photo Library;
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
Copyright © 2016 The Open University