6 The reaction of alcohols with nitric acid – more explosives
As you saw in the last section, to a first approximation, the behaviour of organic functional groups is unaffected by the larger environment of the molecules in which those groups are set. A good example is the reaction of some alcohols with nitric acid, HNO3 (or HONO2), to give so-called nitrate esters (once again focus on the principles being discussed and don’t worry about the names of the compounds):
Thus, hexan-1-ol (Structure 5.1) yields hexyl nitrate, CH3CH2CH2CH2CH2CH2—O—NO2.
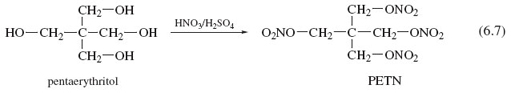
Two organic molecules that contain more than one alcohol functional group are glycerol (Reaction 5.17), made by heating natural fats or oils with sodium hydroxide, and pentaerythritol (Reaction 5.18).
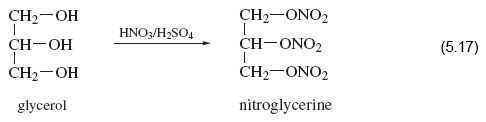
These reactions show how a mixture of concentrated nitric and sulfuric acid replaces all of the —OH groups with —O—NO2 groups, leaving the rest of the molecules unchanged. Thus reinforcing the point made earlier, that reactivity tends to be concentrated at specific sites in a molecule.
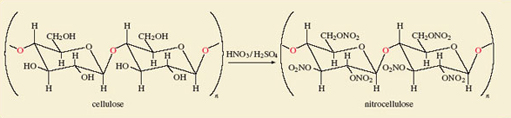
Finally, consider cotton, whose fibres consist of the polymer cellulose – a polymer being a very large molecule made up of many repeated building blocks.
Cellulose is made of glucose molecules linked via oxygen atoms to form long chains. Two molecules joined this way are shown in brackets on the left hand side of Figure 3 and this unit repeats many times. The linking together of these groupings into long chains is indicated by n, the value of which varies, but may be as large as 2000.
Figure 3 shows that despite this polymeric situation, all of the —OH groups can still be replaced by —O—NO2 groups through a reaction with mixed nitric and sulfuric acids.
The products of Reactions 5.17, 5.18 and Figure 3 are called nitroglycerine, pentaerythritol tetranitrate (PETN) and nitrocellulose (also known as guncotton), respectively. They are three important explosives, and the synthesis of each serves to illustrate the reactivity of functional groups relative to the rest of the reactant molecule.
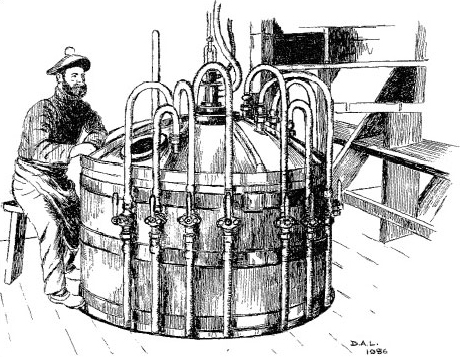
Alfred Nobel (1833-1896) made his fortune through the manufacture of high explosives. Nobel appears here at the controls of the equipment that he invented for the manufacture of nitroglycerine. As shown in Figure 4, the dangerous nature of the work is revealed by the one-legged stool on which he sits. It protects the operator from the mortal dangers of falling asleep on the job!
But what makes functional groups such as —OH so much more reactive than the carbon-hydrogen skeleton to which they are attached? This is considered in the final section.