Life in the Palaeozoic
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Thursday, 25 April 2024, 9:59 AM
Life in the Palaeozoic
Introduction
The Palaeozoic Era was a very important time in the history of life. Using evidence from fossils, we start by looking at the Cambrian explosion, when many forms of animal life first appeared about 545 million years ago. Then we move on to study creatures living in the Ordovician seas, including the extinct trilobites. Next, we'll investigate the invasion of land by plants and invertebrates that occurred in the Silurian Period, and look at life in Silurian seas. You'll also learn about the Devonian Period, when vertebrates first moved onto land. The course finishes with a brief outline of vertebrate evolution.
This OpenLearn course provides a sample of level 1 study in Environment & Development
Learning outcomes
After studying this course, you should be able to:
describe some key events in the evolution of life during the Palaeozoic Era, such as the first appearance of major groups of invertebrates and vertebrates, and the invasion of the land
identify some common types of fossil organisms that were living in Palaeozoic seas, and comment on their likely environment and geological age
make inferences from fossils about the biology and mode of life of some Palaeozoic organisms.
1 The Cambrian explosion
1.1 A burst of evolution
One of the most important events in the history of life began about 545 million years (Ma) ago, i.e. some four billion years after the origin of the Earth, and over 3.3 billion years after the origin of life. The term 'Cambrian explosion' reflects a sudden burst of evolution, when a wide variety of organisms, especially those with hard, mineralised parts, first appear in the fossil record. Thus began the Phanerozoic Eon - 'the time of visible life' - and the Palaeozoic Era.
As part of this course you will often be referred to a pdf file taken from Douglas Palmer's Atlas of the Prehistoric World.
Before going any further, click on 'View document' below and read pages 58-61 from Douglas Palmer's Atlas of the Prehistoric World.
At the start of the Cambrian Period, body fossils with hard shelly parts became abundant for the first time. Actually, the very first evidence of preservable hard parts comes from some tiny fossils, including tubes made of calcium carbonate about 0.2 mm across, found in late Precambrian strata about 550 Ma old. The unknown organisms that produced them were living at the same time as the Ediacaran fauna.
SAQ 1
What, in general, was different about the size of fossils with hard shelly parts in the earliest Cambrian, compared with late Precambrian life such as the Ediacaran organisms?
Answer
The fossils with hard shelly parts were much smaller (Atlas, p. 60).
A wide variety of small (1-2 mm) shelly fossils appeared in the earliest part of the Cambrian Period - assorted shapes such as tubes and cones, as well as spines, scales, and knobs (Atlas, pp. 58-59). It's often difficult to tell whether a fossil is the complete skeleton of a single organism or an isolated part of some larger creature. The soft tissues associated with these hard parts are almost entirely unknown, and the reconstructions of soft parts shown in the Atlas (pp. 60-61) are conjectural.
SAQ 2
What seems likely to have been the main stimulus for the acquisition of hard parts by organisms in the earliest Cambrian?
Answer
The need for protection (Atlas, pp. 60-61).
Protection from predation or other damage is certainly consistent with the first appearance of defensive structures such as spines and scales, and tubes and conical shells that could protect vulnerable soft parts inside. There are, however, a number of other possible reasons why hard parts first evolved, as discussed in the Atlas, p. 61, some or all of which may have been involved.
SAQ 3
A greatly increased variety of types of trace fossils, especially burrows of soft-bodied animals, are found around the start of the Cambrian Period. What is the significance of this finding?
Answer
It reflects the evolution of more complex patterns of activity and behaviour, some of it probably related to the avoidance of predators.
Although environmental changes were occurring (such as a rise in global sea level (Atlas, p. 58), there is, to date, little evidence of special, widespread environmental changes that could have directly triggered the Cambrian explosion. Whatever the causes, once triggered, a wide range of ecological opportunities presumably became available for exploitation, promoting the rapid evolution of new, quite different types of animals. By about 530 Ma, most of the animal phyla that are in existence today had appeared. Not surprisingly, a few entirely soft-bodied phyla living today have no known fossil record, so we don't know when they evolved. Evidence from genetics suggests that some animal phyla had diverged from each other much earlier than the start of the Cambrian explosion, but the timing remains uncertain.
Many of the newly evolved Cambrian phyla show organisation of the body into specialised areas - especially a head end with food-trapping and sensory organs, a tubular gut and limbs. There is no doubt that many Cambrian animals were equipped with adaptations for preying on other animals, and were able to pursue food much more actively than could the Ediacaran fauna - such as by scuttling over the sea floor, swimming actively, and burrowing. Note that all forms of life (except possibly a few algae) were as yet confined to the sea.
Although many types of the small shelly fossils disappear from the fossil record soon after the start of the Cambrian, some are thought to have been molluscs at an early evolutionary stage, in which case they did leave descendants.
1.2 The Burgess Shale
High in the Canadian Rockies is exposed a deposit of middle Cambrian age, about 530 Ma old, called the Burgess Shale. It contains the fossils of animals that lived on a muddy sea floor, and which were suddenly transported into deeper, oxygen-poor water by submarine landslides. Their catastrophic burial has given us an exceptional view of Cambrian life. Not only have animals with hard shelly parts been preserved but entirely soft-bodied forms are also preserved as thin films on the sediment surface.
Before going any further, click on 'View document' below and read pages 62-65 from Douglas Palmer's Atlas of the Prehistoric World.
Some of the most common Cambrian fossils, which appear immediately after the first shelly fossils, are trilobites. These were a group of exclusively marine arthropods, members of the enormously diverse phylum of animals with jointed, external skeletons that today include forms such as crabs, lobsters, insects and spiders. The trilobite fossils of the Burgess Shale are like many trilobites found elsewhere but exceptional in that not only is the main part of their outer skeleton (or exoskeleton) preserved, but so too are their appendages such as antennae and legs (see, for example, those of Olenoides, Atlas, p. 63). Elsewhere, trilobite appendages are extremely rare as they were poorly mineralised. We will study trilobites in more detail shortly. Other types of arthropods, especially ones lacking well-mineralised exoskeletons (such as Marrella, Atlas, p. 64), are particularly abundant in the Burgess Shale.
Only about 15 per cent of the 120 genera present in the Burgess Shale are shelly organisms such as trilobites and brachiopods that dominate typical Cambrian fossil assemblages (fossils that occur together) elsewhere. The shelly component was therefore in a minority, and organisms with hard parts probably formed less than 5 per cent of individuals in the living community.
SAQ 4
If the soft-bodied fossils of the Burgess Shale are taken away, all that remains is a typical Cambrian assemblage of hard-bodied organisms. Why is this important to bear in mind when trying to interpret other Cambrian fossil assemblages?
Answer
The other Cambrian assemblages may also have been dominated by soft-bodied animals, even if the only fossils they now contain are of hard-bodied ones.
Another important revelation of the Burgess Shale lies in the wide diversity of animal types that were around in middle Cambrian time, about 530 Ma ago. There are representatives of about a dozen of the phyla that persist to the present day. One form closely related to early arthropods was Anomalocaris, the largest known Cambrian animal, some individuals of which may have reached two metres in length. Its extraordinary jaw (Atlas, p. 65) consisted of spiny plates encircling the mouth, which probably constricted down on prey in much the same way that the plates of an iris diaphragm cut down the light in a camera. This fearsome mouth is seen in place in the reconstruction of the closely related Laggania (Atlas, pp. 64-65). Note that the colours of organisms shown in this and other such reconstructions are conjectural.
About a dozen types of Burgess Shale fossils have been said to be so unlike anything living today and so different from each other that, had they been living now, each would have been placed in a separate phylum. With further study, however, the relationships of these puzzling animals (such as Hallucigenia, Atlas, p. 63) are becoming clearer. It seems that some Burgess Shale forms are hard to classify simply because the boundaries between major categories of animal life were still blurred shortly after the Cambrian explosion. In other words, by mid Cambrian time, there still had not been enough time for some groups to have diverged sufficiently from their recent common ancestors to be distinctly different.
Burgess Shale-type faunas have been found in about 30 sites ranging from North America and Greenland, to China and Australia. The wide range of animals they contain seems to reflect an unpruned 'bush of diversity' resulting from the Cambrian explosion. Not long after, though, extinction lopped off some of the branches, leaving phyla with the relatively distinct features that have remained to this day.
1.3 An overview of animal phyla
We have already met quite a few different animal phyla, and it's useful to get an overview of all the ones commonly found in the fossil record and their mode of life before studying some in more detail. Except for a few soft-bodied phyla with very poor fossil records, it is clear that all the animal phyla had appeared by the Ordovician Period.
Modes of life
The following terms are very useful for describing the mode of life and the environmental setting of organisms. The terms are explained here as they are applied to marine organisms, but they are also sometimes applied to organisms living in lakes.
benthic - animals and plants that live on the sea floor; collective noun: benthos.
pelagic - animals and plants that live above the sea floor. They may be either nektonic (animals only) or planktonic (animals and plants).
nektonic - animals that swim actively (e.g. fish, squid); collective noun: nekton.
planktonic - animals and plants that drift passively or swim feebly, mainly in the surface waters of seas; collective noun: plankton. The term includes phytoplankton (photosynthetic organisms, mostly algae) and zooplankton (mostly microscopic animals, including larvae of larger ones, but also some macroscopic animals that are readily visible with the naked eye, e.g. jellyfish).
epifaunal - animals that live on the sea floor, either on soft sediment, or attached to rocks, seaweed, etc. (sessile), or that move over the sea floor (vagrant); collective noun: epifauna.
infaunal - animals that live within sediment, often in burrows or borings into harder material; collective noun infauna.
Like most classifications involving living organisms, some invertebrates do not fit neatly into these categories, e.g. the common prawn buries itself in sediment during the day (i.e. is infaunal), but at night emerges to join the epifauna as it feeds. Other epifaunal animals bury themselves during low tide.
The following text gives some key points about the important animal phyla most commonly found in the fossil record. The age range and mode of life of some common groups are given, and phyla that are microscopic throughout life are excluded. Figure 1 shows typical fossil representatives of some of the phyla.
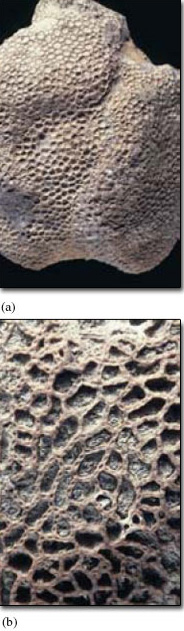
Porifera. Sponges. Cambrian to Recent. Mainly marine; some freshwater. Sessile. The simplest multicellular animals, sponges lack definite tissues and organs, e.g. they have no nervous system. They have a skeleton of calcium carbonate, silica, or, as in some bath sponges, horny organic material. Water passes in through the sponge's many surface pores, often to the central cavity of a sack-like body, and out through a large hole at the top. Some have a stalk (Figure 1a), others are encrusting and irregular in shape. Sponges feed by filtering off minute organic particles from the water. Sponges are locally abundant fossils, especially in Cretaceous rocks, where they are very commonly enclosed in flint nodules in the Chalk.
Cnidaria (pronounced with a silent 'C': 'nigh-dare-ee-a'). Late Precambrian (Ediacaran) to Recent. Almost entirely marine. Cnidarians have a central mouth around which are stinging tentacles for catching prey. By far the most important fossil group are the entirely marine, generally benthic, corals, which secrete a skeleton of calcium carbonate below the soft, anemone-like parts at the top. Corals may be either colonial (with many genetically identical, linked individuals sharing a skeleton) or solitary individuals. There are three main groups of corals: rugose corals (solitary, Figure 1b, or colonial), Ordovician to Permian; tabulate corals (always colonial), Ordovician to Permian; and scleractinian corals, sometimes also called hexacorals (solitary or colonial), Triassic to Recent. Sea anemones and jellyfish are also cnidarians, but being soft-bodied these groups are much less common in the fossil record.
Bryozoa. Bryozoans. Ordovician to Recent. Sometimes called 'moss animals'. Normally marine, rarely freshwater. Sessile, tiny animals which live in colonies, with a skeleton usually of calcium carbonate. The colonies vary in shape; some are encrusting sheets ('sea mats'), others may be delicate net-like fronds (Figure 1c) or branching twigs. Each colony consists of a few to thousands of interconnected individuals. When feeding, tentacles filter microorganisms from the water. Bryozoans often occur as fossils among the diverse fauna of reefs. Some bryozoans look rather like small corals; a few species can look a little like graptolites (see below).
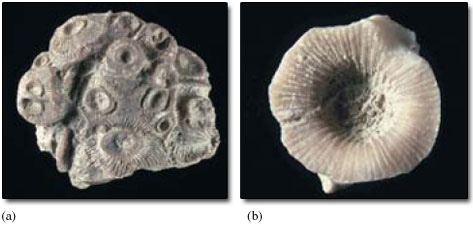
Brachiopoda. Brachiopods (pronounced 'bracky-o-pods'). Cambrian to Recent. Entirely marine, benthic animals. Sometimes called 'lamp-shells' (after their resemblance to Roman oil lamps). Brachiopods are typically 2-5 cm long, but they range in size from a few mm to as much as 30 cm. The shell, which encloses the soft tissues, has two parts, called valves. One valve is almost always larger than the other. Many brachiopods are attached to the sea floor by a stalk of horn-like or fleshy material, the pedicle; in fossil brachiopods the presence of this pedicle is indicated by a hole passing through the larger of the two valves (Figure 1d). Some are free-lying on the sea floor, and a few are cemented or attached by spines. In most brachiopods the shell is composed of calcium carbonate, though some are phosphatic. They feed by drawing water into the shell and filtering off food particles with a complex feeding device called a lophophore. Brachiopods are much less abundant and diverse today than during the Palaeozoic and Mesozoic. They are the commonest fossil in many Palaeozoic shallow marine limestones and shales. About 20 species occur today off the British Isles, mostly in deeper waters, and are rarely seen.
Mollusca. Molluscs. Cambrian to Recent. Mainly marine. A very diverse phylum, perhaps numerically the most abundant large invertebrates in the fossil record. There are shelled and unshelled forms. Although at first the six or so living classes may seem unrelated, they represent evolutionary variations on the same theme - the molluscan bodyplan. Three classes, each of which range from Cambrian to Recent, are particularly important, both as fossils and today: the Bivalvia - bivalves (e.g. cockles, mussels and oysters) (Figure 1e); the Gastropoda - gastropods (e.g. slugs and snails); and the nektonic Cephalopoda - cephalopods (e.g. squid, cuttlefish, octopus and nautilus). Most bivalves are marine (vagrant to sessile benthos), though some are freshwater. The majority of gastropods are aquatic, and most of these live in shallow seas, but they are also widespread in freshwater and on dry land. Cephalopods are (and have been) entirely marine, and are the most highly evolved molluscs. The most important fossil cephalopod groups are all forms with chambered shells - the nautiloids (Cambrian to Recent), the familiar spiral-shelled ammonites (Triassic to Cretaceous), and the bullet-shaped belemnites (Jurassic to Cretaceous).
Echinodermata. Echinoderms. Cambrian to Recent. Entirely marine. Most are benthic. Many are vagrant, some are sessile, and a few are free-swimming (nektonic). Most echinoderm skeletons are made of many porous plates of calcite (calcium carbonate) which are very thinly covered with soft tissue. Multipurpose, extendible tentacles called tube feet emerge to the outside, and are used especially in feeding, respiration and locomotion. In many forms there is a distinctive five-rayed arrangement of plates and tube feet. Echinoderm means 'spiny skin', referring to the fact that some groups have spines or hard, warty bumps projecting from the surface. The most common fossil groups are sea urchins (echinoids), Ordovician to Recent, and sea lilies (crinoids), Cambrian to Recent. Two other well-known living groups, both Ordovician to Recent, are starfish (asteroids) and brittle stars (ophiuroids). There are also several extinct groups.
Arthropoda. Arthropods. Cambrian to Recent. The largest phylum of animals, with a great diversity of morphology and mode of life; living forms are found in most possible habitats in water and on land. A partial list of groups includes crustaceans (crabs, lobsters, barnacles and shrimps), insects, millipedes, centipedes, spiders, king crabs, scorpions, mites and several extinct groups, of which the entirely marine, extinct trilobites (pronounced 'try-lo-bites') are the most important. The most characteristic features of the phylum are the hard outer coating (exoskeleton) which is divided into segments, and the paired, jointed appendages which vary in number and function. The exoskeleton, usually of chitin (a strong, lightweight organic material), may be further strengthened by calcium carbonate or calcium phosphate, increasing the preservation potential. Growth occurs during periodic moulting when the exoskeleton is shed and a new, larger one is formed.
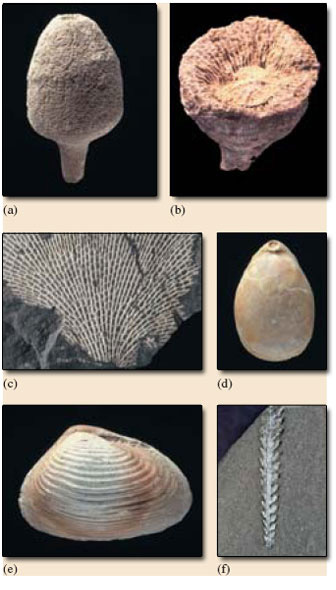
Hemichordata*. Hemichordates. Cambrian to Recent. By far the most important fossil group are the graptolites - extinct, entirely marine colonies confined to the Palaeozoic Era, particularly abundant in Ordovician and Silurian rocks where they are very useful zone fossils. Many look like saw blades a few centimetres long on the rock, with 'teeth' on one or both sides of the 'saw' (Figure 1f). The 'teeth' were actually tiny cups that housed individuals which made up the colony and possessed filter-feeding tentacles. Some graptolites were benthic and sessile, but most were pelagic, either drifting or possibly swimming feebly as part of the zooplankton.
*Some biologists place the hemichordates as a subphylum within the Phylum Chordata.
Chordata. Chordates. Cambrian to Recent. All chordates possess a notochord (a flexible rod running along the length of the body). Minor living chordate groups, lacking a vertebral column (backbone), include sea squirts and the lancelet. The major group are the vertebrates, which have bony or cartilaginous skeletons and a head. Today, vertebrates include five classes: fish (Cambrian to Recent), amphibians (Devonian to Recent), reptiles (Carboniferous to Recent), mammals (Triassic to Recent) and birds (Jurassic to Recent). Important extinct reptile groups include the land-dwelling dinosaurs (Triassic to Cretaceous), the marine ichthyosaurs (Triassic to Cretaceous) and plesiosaurs (Jurassic to Cretaceous), and the flying pterosaurs (Triassic to Cretaceous). Vertebrate fossils are relatively rare compared with invertebrates, and are usually only fragments.
1.4 The origin of the vertebrates
Vertebrates such as ourselves are by definition animals with a backbone (or vertebral column, paired limbs, a skull and various other structures. Until recently vertebrates were thought to extend back only into late Ordovician times, some 450 million years ago. At this time fossils of strange-looking fish with bony headshields, such as Sacabambaspis (Atlas, pp. 70-71), appear in the fossil record. These jawless fish (called agnathans) are only very distantly related to the sole living agnathans, the lampreys and hagfish. However, these Ordovician creatures are already highly evolved and clearly had yet more ancient ancestors.
To understand what such ancestors might have been like we need to consider the backbone - the fundamental vertebrate feature - in a bit more detail. The precursor to the backbone is a stiffening rod, called the notochord, details of which we know from the study of developing vertebrate embryos and the few surviving animals which have retained a notochord, such as the lancelet Branchiostoma.
Before going any further, click on 'View document' below and read pages 66-67 from Douglas Palmer's Atlas of the Prehistoric World.
SAQ 5
What is the main function of the notochord?
Answer
It lengthens and stiffens the body, allowing the blocks of muscles to flex the body sideways into zigzag bends for swimming (Atlas, p. 66).
The fundamental chordate characteristic of a notochord (Section 1.3) has now been identified in various Cambrian fossils, such as Pikaia from the Burgess Shale (Section 1.2).
SAQ 6a
Why is Pikaia classified as a chordate but not a vertebrate?
Answer
It has a notochord but not a backbone, nor other vertebrate features such as paired limbs (Atlas, pp. 66-67).
The extinct conodonts (late Cambrian to end Triassic in age) have been shown to possess not only a notochord but also tooth structures and paired eyes, which seem to suggest that they were more advanced than chordates such as Pikaia and close to the earliest vertebrates. (You will read about conodonts shortly on pp. 70 of the Atlas.) Some early Cambrian fossils recently found in China are thought to preserve all the basic chordate features plus some more advanced vertebrate ones, namely gills as well as paired eyes. If so, then it is clear that some animals close to the vertebrate bodyplan had already appeared by early Cambrian times, and chordate ancestry probably reaches back into the late Precambrian.
SAQ 6b
Humans belong to the vertebrate group known as mammals. Using Section 1.3, place mammals and the other four main vertebrate groups in order of their evolutionary appearance.
Answer
The earliest generally recognised group of vertebrates to appear in the fossil record are fish, followed by amphibians, reptiles, mammals and finally birds.
2 The Ordovician seas
Before going any further, click on 'View document' below and read pages 68-71 from Douglas Palmer's Atlas of the Prehistoric World.
Collecting seashells on an Ordovician beach would have been a rather curious experience. Whilst most shells were made of similar materials to those found on a modern beach, the detailed form of many would have been quite unfamiliar, and all the species have long been extinct.
Have a look at the panoramic illustration on pp. 70-71 of the Atlas. From this and the Atlas, pp. 68-71, think about which organisms appear most unfamiliar to you.
There are many organisms that probably seem unfamiliar, and when reading about them you may want to refer to Section 1.3. Brachiopods, e.g. Strophomena, are superficially clam-like animals, and although not extinct, are much rarer now than in the Palaeozoic; most people have never seen a living one. Graptolites, e.g. Orthograptus, are extinct colonial animals that mostly drifted in the ocean currents (Figure 2). Trilobites, e.g. Triarthrus, are extinct marine arthropods. Conodonts, e.g. Promissum, are extinct, and jawless (agnathan) fish, e.g. Sacabambaspis, are extinct except for hagfish and lampreys (see Section 1.4). The straight-shelled nautiloid cephalopods, e.g. Endoceras, are extinct and only distantly related to today's Nautilus, which has a spiral shell. The groups still very much with us include horseshoe crabs, snails (gastropods), e.g. Cyclonema, and corals (although Palaeozoic corals were significantly different from modern corals). Bivalves (not shown) were much rarer in the Ordovician seas than they are today.
All this fauna was marine. Very little, if any, animal life had made it out of the sea onto dry land by the end of the Ordovician. Trace fossils of an unknown, possibly millipede-like animal (Atlas, p. 71) are rare evidence that invertebrates were exploring the edge of the land by a freshwater route. There is fossil evidence that bryophyte-like plants and fungi had begun to colonise land environments back in Ordovician times. Apart from a small advance guard, however, the main invasion of freshwater and land environments by plants and animals did not really get going until the Silurian Period.

3 The Silurian Period and the invasion of the land
Before going any further, click on 'View document' below and read pages 72-75 from Douglas Palmer's Atlas of the Prehistoric World.
SAQ 7
What global event had reduced global sea level at the end of Ordovician times, drastically affecting shallow marine organisms, and leaving the diversity of early Silurian life severely curtailed?
Answer
The global climate descended into an ice age, locking up ocean water in the polar ice caps, exposing much of the continental shelves above sea level, eliminating many shallow marine habitats and driving many species to extinction (Atlas, p. 69). (Sea level probably fell by about 100 m, not 330 m as stated in the Atlas.)
Recent research shows that minor glaciations continued into the early Silurian, but after a while the oceans became warmer again and sea levels rose. It took several million years for marine life to recover. At first, stromatolites were relatively common, apparently because the organisms that normally suppressed them had suffered severely, but by mid Silurian times, vast coral reefs were established in tropical waters, promoting a rich diversity of organisms. The growth of corals with their tough, mineralised skeletons turns flat sea beds into complex 3-D topographies, baffling ocean currents, and providing sheltered surfaces and new ecological niches. We will soon study some of the organisms that thrived in such environments.
Meanwhile, the animals and plants that were invading the land faced all sorts of environmental challenges to which they had to adapt. For example, if a marine plant cell is directly surrounded by fresh water, the water tends to flow into it by the process of osmosis, causing it to burst. Alternatively, if the cell is directly surrounded by air, it loses all its water, just as seaweeds become hard and crisp when stranded above high tide and exposed to the wind and the sun. So, to survive in air, plants had to acquire an effective outer coat to keep the right amount of water in. They also had to evolve small, controllable pores to enable gases to be exchanged through this coat. Expressed this way, it is all too easy to give the impression, quite wrongly, that such innovations could be achieved intentionally, almost as if by some directed effort. On the contrary, as in all evolutionary explanations, natural selection would have favoured those organisms that were, by chance, better adapted to these new environmental challenges.
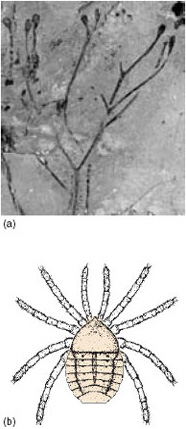
Without the buoyancy provided by immersion in water, adaptations in both plants and animals were needed to support a body on land against the pull of gravity. The relatively high density of water provides much greater support for the bodies of marine organisms than does less dense air - hence the expression 'like a fish out of water'. To grow up off the land surface, plants had to develop groups of special plumbing cells to conduct water, nutrients and the products of photosynthesis around their bodies. Only about 4 cm tall, Cooksonia (see Figure 3a and illustration, Atlas, p. 75) lacked leaves, and sent short, forking shoots upward to capture sunlight and release spores (reproductive cells) into the wind. These first true vascular land plants with specialised cells for carrying fluids around an upright plant were still dependent on water for reproduction, and lived in swamps and on riverbanks and floodplains. The earliest movement onto land, for both plants and animals, seems to have been through the medium of fresh, as opposed to saline, water.
Air, being a gas, has a low capacity to store heat compared with seawater and, to survive, life on land must be able to withstand relatively large temperature fluctuations as well as higher amounts of ultraviolet radiation from the Sun. Exchange of gametes during reproduction is also much easier in water where male gametes can swim to fertilise the female ones. The animals that, by chance, were best adapted to life on land were the arthropods. They were very strong for their size, and already had an almost waterproof outer skeleton that was resistant to damage by ultraviolet light. Numerous paired and jointed limbs with internal muscles helped overcome gravity and allow movement over uneven terrestrial surfaces. However, the arthropods could not have invaded the land if there had not already been some supply of food there.
SAQ 8
According to the Atlas (p. 74), what are the first arthropods believed to have eaten?
Answer
The first arthropods were probably eating the decaying remains of plants such as Cooksonia.
Once the greening of the land had begun, small millipedes and wingless insects were apparently tempted onto it to eat the rotting plant debris, and they and their remains were eaten in turn by predatory or scavenging carnivorous arthropods such as centipedes, scorpions and small spider-like creatures (Figure 3b).
4 Life in the Silurian sea
4.1 Trilobites
As we've seen, the Cambrian explosion left the seas teeming with a huge variety of animals. In the following activity you will study some of the marine life at one particular time in the Palaeozoic Era - the middle part of the Silurian Period, 430 Ma ago. You'll look in detail at some fossils which come from a deposit in the UK called the Wenlock Limestone, famous for its many beautiful fossils. The Wenlock Limestone crops out mainly around Birmingham and the borders of Wales.
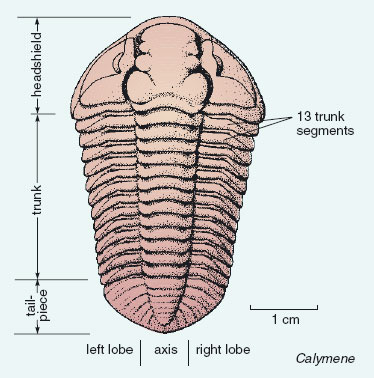
Figure 4 shows a trilobite from the Wenlock Limestone, called Calymene ('kal-iminny').
Trilobites were a major group of entirely marine arthropods that thrived in the seas of the Palaeozoic Era and eventually became extinct in the Permian Period. The arthopods are an immensely diverse phylum (see Section 1.3). Their external jointed skeleton (exoskeleton) forms a robust armour-plating for the body, though it is flexible at the joints to allow movement.
SAQ 9
Being arthropods, how did trilobites get around the problem of ytheir growth being constrained by an external skeleton?
Answer
Trilobites, like other arthopods, grew by moulting, i.e. periodically casting off their rigid shell, and secreting a new, larger one.
Trilobites are so named because they have three lobes running up and down their length - a central axis, and two lobes: one on either side. They are also divided cross-ways into a headshield, a trunk, and a tailpiece. Have a preliminiary look at the fossil in Figure 4 to see if you can identify these divisions. The trunk has a number of separate segments, and the tailpiece is made of a single plate. The more formal names for the three main divisions of the trilobite body, often used elsewhere, are as follows:
headshield = cephalon; trunk = thorax; tailpiece = pygidium.
Trilobites also had appendages such as antennae and legs, but these are extremely rarely found. An example where legs are preserved is shown on p. 63 of the Atlas.
SAQ 10
What does the rarity of trilobite appendages suggest about their structure?
Answer
They were not so durable, being less mineralised than the components normally found (headshield, trunk and tailpiece), and soon decayed like other soft tissues.
Because trilobites cast off their shell during moulting, most trilobite fossils are actually shed shells, rather than carcasses (dead animals). The trilobite's hard outer shell needed places of weakness along which it could break apart during moulting, a bit like having weak areas between the pieces of a bar of chocolate. There were lines of weakness between the headshield and the front of the trunk; between any two trunk segments; and between the end of the trunk and the front of the tailpiece. Trilobites also had one or more lines of weakness within the headshield. Figure 4 shows these lines of weakness for Calymene; they allowed two side pieces to detach from the larger, central part of the headshield.
Many trilobites had eyes. In Calymene (Figure 4), they are at the cresent-shaped areas on the detachable side pieces either side of the central part of the headshield. The eyes were rather like the compound eyes of a modern fly (another arthropod) in having many lenses. Each trilobite lens was made of calcite, and its preservation potential was as good as the rest of the skeleton (also made mostly of calcite). Trilobites had the earliest recorded eyes in the animal world.
The presence of well-developed eyes suggests that wherever the trilobites were living there was enough light to see by, and, as light fades with depth, the water is unlikely to have been very deep.
It is likely that Calymene spent more time scuttling around on the sea floor than swimming high up in the water. It probably rested on the sea floor, stretched out in the same way as in Figure 4. These trilobites probably ate small organisms and organic debris on or near the sea floor.
Some trilobites could roll up, tucking the tail snugly underneath the head. Figure 5 shows a specimen of Calymene that has rolled itself up into a ball, like a pill bug (a type of woodlouse, another arthropod) can do. The flat, outer edges of the trunk segments had to slide over each other to enable the trilobite to roll up.
SAQ 11
Why might the ability to roll up be useful?
Answer
It would protect any soft parts underneath the hard external skeleton from attack by predators, damage during storms, etc.
By studying fossils one can observe various features of the morphology of an extinct organism, and infer aspects of its mode of life and environment. We can do this by comparing observed features with what is known of living relatives and similar fossils. In practice, when studying fossils one would also take other information into account, such as evidence from the sediment enclosing the fossil, and so on. The result is always an interpretation that reflects the balance of probabilities from the available information, rather than the certain truth.

4.1.1 More on trilobites
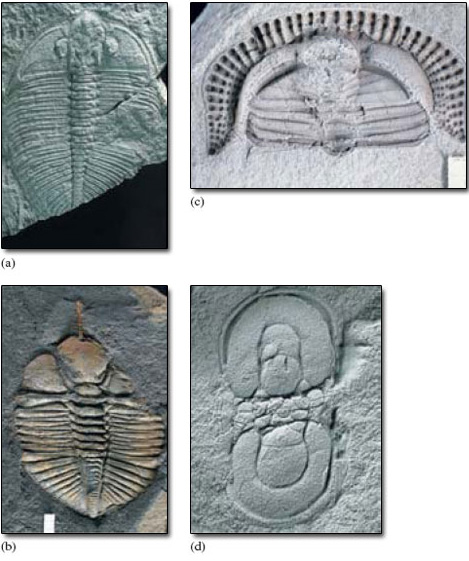
Many thousands of trilobite species are known, mostly from Cambrian to Silurian rocks, and all were confined to the Palaeozoic Era. By the time trilobites became extinct in the late Permian, their diversity had dwindled to a small number of species, and the group was long past its peak. The variation in trilobite form is enormous, but the basic three-lobed division of the exoskeleton is always present. The number of trunk segments varies from 2 to 40. Not all have eyes. Most are about 2-10 cm in length but some are 1-2 mm and a few species grew to nearly 1 m long. The majority of trilobites lived on or near the floor of shallow seas, but some trilobites swam in the surface waters of the open ocean, and some were adapted to low concentrations of oxygen in water hundreds of metres deep. How they reproduced is not clear. Figure 6 shows a range of trilobites from the Ordovician Period.
4.2 Crinoids
Figure 7 shows the fossilised remains of a type of echinoderm called a crinoid ('cry-noyed'). Although crinoids occur today, they were far more common in the Palaeozoic and Mesozoic Eras. Most crinoids feed by bending their umbrella-like arrangement of flexible appendages (called 'arms') downstream so as to catch a current, rather as in an umbrella being caught in the wind. Tube feet (multipurpose tentacles) on the arms gather food particles suspended in the water, which are then wafted by small hair-like threads in grooves along the arms to the central mouth. Most ancient crinoids lived gregariously in shallow, current-swept areas, free of muddy sediment that would otherwise have tended to kill them by clogging their feeding mechanism.
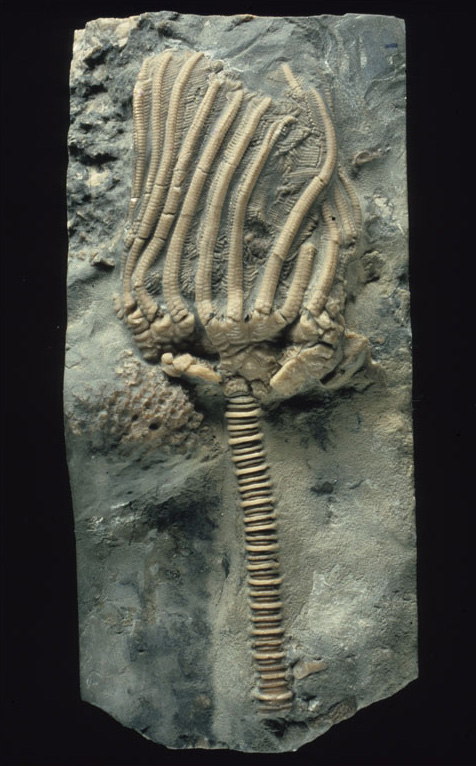
Most ancient crinoids were attached to the sea floor by a stem or stalk with a root-like holdfast. The mouth and gut were situated in an enclosed cup at the top of the stem. In life, the stem was fairly flexible, like the arms. The majority of living crinoids do not have a stem, and are capable of creeping around or even swimming. The few surviving stemmed forms generally live in deep water. Although they are animals, crinoids with stems look at first so like plants that they are often informally called 'sea lilies'. Shortly after death, the tiny organic fibres that alone hold the calcite plates together rot away, often causing the crinoid to disintegrate into separate plates that can be readily dispersed by currents or the movement of other organisms. In some ancient environments where crinoids were abundant, rocks have formed that are largely composed of isolated plates and stem fragments (Figure 8).
Crinoids have an arrangement of five 'rays', each of which carries one or more arms that together form an efficient food-gathering structure. Attached to each arm are many fine side-branches called pinnules, each made of tiny plates, which are also part of the food-gathering apparatus.

4.3 Corals
Corals are especially abundant in the Wenlock Limestone.
SAQ 12
According to Section 1.3, which two main groups of corals, each confined to the Palaeozoic Era, might you expect to find in the Silurian Period?
Answer
Rugose corals and tabulate corals.
SAQ 13
Which of these two coral groups only forms colonies?
Answer
The tabulate corals are always colonial (whereas the rugose corals may be either colonial or solitary).
Figure 9 shows two rugose corals. When alive, the Acervularia colony in Figure 9a would probably have looked like a bunch of sea anemones, with each of them sitting in one of the bowl-shaped hollows of their calcite skeletons. The skeleton secreted by an individual, whether part of a colony or not, is called a corallite. In rugose corals, each corallite is usually divided by a series of conspicuous radial partitions, called septa (Figure 9). The corallites in a colony such as Acervularia share adjacent walls. In the centre of many corallites of Acervularia is a single, bowl-shaped hollow. Within some corallites there are one or more smaller hollows. These little hollows have the same basic arrangement of septa as in the larger corallites: they represent new individuals formed by the splitting up of their parent, enabling the coral colony to grow upward and outward by a process of asexual reproduction.
Tabulate corals are distinguished by having much smaller corallites than those of rugose corals, and septa are either absent or short and inconspicuous. Tabulate coral colonies may take various forms, including massive (Figure 10a) or chains (Figure 10b).
Some Wenlock Limestone corals are shown on pp.74 and 75 of the Atlas; see if you can distinguish the solitary corals from colonial ones. The coral at the bottom of the group on p. 75 is a tabulate coral called Heliolites.
SAQ 14
What sort of environments do corals tend to be associated with today?
Answer
Images of Australia's Great Barrier Reef and tropical islands like the Bahamas probably spring to mind (though not all corals live in shallow waters).
SAQ 15
According to Section 1.3, what is the main group of modern corals, and what is their age range?
Answer
Scleractinian corals; Triassic to Recent.
Figure 11 shows some examples of modern coral skeletons, both solitary and colonial. Like rugose corals, scleractinians usually have conspicuous septa. However, unlike both rugose and tabulate corals, the skeleton of scleractinians is made of aragonite, not calcite. Although it is also a form of calcium carbonate, aragonite tends to alter to calcite or dissolve away, so that Mesozoic and Cenozoic corals (i.e. scleractinians) are often less well preserved than Palaeozoic ones.
Most, but not all, scleractinian corals today grow in warm, clear, shallow seas. The same seems to have been true of many ancient corals, including Palaeozoic ones. The requirement of many of today's corals for clear, shallow water is mostly because within the corals' soft tissues are tiny algae with which they live in an association of mutual benefit. The algae require clear, well-lit water for photosynthesis. By analogy with the preferences of most modern corals, and what is known of ancient ones, it therefore seems very likely that the water of the Wenlock Limestone sea was warm rather than cold. This is supported by palaeomagnetic evidence showing that at this time Britain was about 25° south of the Equator. Palaeozoic and scleractinian corals are, however, unrelated and there is evidence that Palaeozoic corals lacked a special association with algae.

4.4 Other Wenlock Limestone fossils
Among the other fossils common in the Wenlock Limestone are brachiopods (Figure 12a and b), gastropods (Figure 12c) and bryozoans (Figure 12d). You may need to reread Section 1.3 to remind yourself about various aspects of these groups.
Figure 13 (the course image) is a reconstruction of a typical scene from a Wenlock Limestone environment. See if you can identify trilobites (alive, dead, or moulted pieces of exoskeleton), corals (colonies and isolated individuals), and crinoids among the various forms of life depicted. The cone-shaped squid-like animals are straight nautiloids (cephalopod molluscs, Section 1.3).


5 The Devonian Period
Before going any further, click on 'View document' below and read pages 76-77 from Douglas Palmer's Atlas of the Prehistoric World. Do not worry too much about all the different names of fish groups in this, the 'Age of Fishes'.
Environmental change is known to have a significant impact on the evolution of life. For example, widening oceans generate barriers between populations and promote increasing genetic divergence between them over time. Narrowing oceans, rising mountain belts and the formation of large continental areas can also have major effects, causing both evolution and extinction. During the Silurian, the Iapetus Ocean had been narrowing (Atlas, p. 72), allowing the progressive mixing of marine organisms that had previously been separated by a wide oceanic barrier. By the Devonian, the continental areas of Laurentia, Avalonia and Baltica had amalgamated to form a large land mass, with huge rivers and lakes that were colonised firstly by jawless fish and then by predatory jawed fish. (Jawed fish had first evolved in the Ordovician (Atlas, p. 71), but only became abundant in the late Silurian and Devonian, evolving into many different marine and freshwater groups.) The rising mountain chains separated river systems and promoted the evolution of different species of fish in different inland waterways. Likewise, land plants were spreading globally, evolving distinct floras in particular regions with different climates.
We now look at one of the most significant developments in the evolution of our vertebrate ancestors - the first tetrapods in the late Devonian.
6 Vertebrates move onto land
6.1 A difficult evolutionary transition
Before going any further, click on 'View document' below and read pages 78-79 and 82-83 from Douglas Palmer's Atlas of the Prehistoric World.
As we saw in Section 3, the move out of water on to land was a particularly difficult evolutionary transition, requiring many adaptations.
SAQ 16
What specific adaptations did vertebrates evolve for living on land, and why were they needed?
Answer
Muscular limbs that lift the body and propel it forward, as movement on land could not easily be achieved with fish-like fins (note that limbless snakes today can move over land, though the skeletons of some snakes retain internal vestiges of limbs possessed by their ancestors); internal lungs, as the gills of fish collapse out of water; 'ears' to hear sound in air as opposed to water; and moist eyes and skin to prevent them drying up in air (Atlas, pp. 78-79).
The earliest four-limbed vertebrates (i.e. tetrapods) are known from late Devonian river and lake deposits in Greenland. Acanthostega and Ichthyostega have a somewhat puzzling mixture of characteristics. They have four jointed, muscular limbs that were originally interpreted as legs for living on land, but they also have long, sideways-flattened, fish-type tails for swimming, a sensory system for detection of vibrations in water and, most important, fish-type gills. The current interpretation therefore sees the limbs as adaptations for life in a particular aquatic environment rather than for life on land. However, these limbs also had very appropriate preadaptations for the tetrapod descendants who did leave the water. By the early Carboniferous, limbs with five digits had become the norm, which has remained so ever since.
SAQ 17
Look at the panorama on pp. 78-79 of the Atlas. How many digits ('fingers' or 'toes') did Acanthostega and Ichthyostega have on each limb?
Answer
Acanthostega had eight digits, Ichthyostega had seven or possibly eight digits.
SAQ 18
What were the earliest tetrapod limbs supposedly used for in this late Devonian aquatic environment?
Answer
The muscular limbs, hands and feet were used for additional propulsion (aiding the fish-like tail), for holding on to plants and perhaps digging for shellfish (Atlas, p. 78).
By early Carboniferous times, fossils from localities such as East Kirkton in Scotland reveal the existence of several different tetrapod groups (Atlas, pp. 82-83). Some of these were still fully aquatic, but others, such as the superficially lizard-like Westlothiana and the salamander-like Balanerpeton, had probably made the transition to a semi-aquatic life. Life in the water was hazardous, with intense competition for food, and one of the selection pressures favouring early tetrapods leaving the water may have been the chance to exploit the abundant food resources on land. However, life on land could also be dangerous, as witnessed, for example, by the scorpion, Pulmonoscorpius, which grew up to 70 cm long.
You might wonder why the word 'tetrapod' is used here instead of amphibian or reptile. Certainly, modern tetrapods are clearly separated into amphibians, reptiles, birds and mammals, largely on the basis of their distinctive reproductive systems. Amphibians breed in water, where they lay unprotected eggs which are externally fertilised. By contrast, reptiles, birds and mammals use internal fertilisation. In reptiles and birds, the embryo develops in fluid surrounded by a protective shell - an egg that can be laid on land. In nearly all mammals, the developing embryo is also surrounded by fluid and a protective membrane, but is retained in the mother's body for some time before birth. Such protective membranes are not confined to the mammals but are also found in reptiles and birds, all of which are grouped as amniotes. The amniotes are therefore more independent of water than amphibians whose embryos are not protected by such membranes and therefore have to develop in water. The problem is that the early tetrapod fossils do not preserve direct evidence of reproductive habits. Also, during early tetrapod evolution there would not have been so clear a separation between amphibians and reptiles as we find today. Only later, when clearly identifiable amphibian or reptilian characteristics emerge in the fossils, is classification easier.
By Carboniferous times terrestrial food chains were already well developed with a great variety of plants such as tree-sized clubmosses, seed plants and tree ferns and smaller, ground-covering ferns. These plants supported a wide variety of invertebrate life, including many arthropods, which provided abundant food for the carnivorous land-living tetrapods.
6.2 An outline of vertebrate evolution
Let's now place the early evolution of tetrapods in perspective by taking an overview of the whole of vertebrate evolution.
Click to view printable version of image
Given the ubiquity of our own primate species, and familiarity with domesticated animals such as cats, dogs, and rabbits, our view of the relative diversity and abundance of the main vertebrate groups tends to be biased towards mammals. However, a tally of living vertebrate species reminds us that mammals are not as dominant as some might imagine. In round terms, the total number of living vertebrate species is over 66 000 (which compares, for example, with some 110 000 species of molluscs and more than 80 000 species of roundworms (nematodes)). Living mammals number around 6000 species, and amphibians around 8000 species, whereas there are some 12 000 reptile species, 11 000 bird species and over 28 000 species of bony fish, and about 800 species of sharks and rays, the cartilaginous fish. (You may want to look up the latest figures online as you read this section, as these numbers are constantly refined.)
SAQ 19
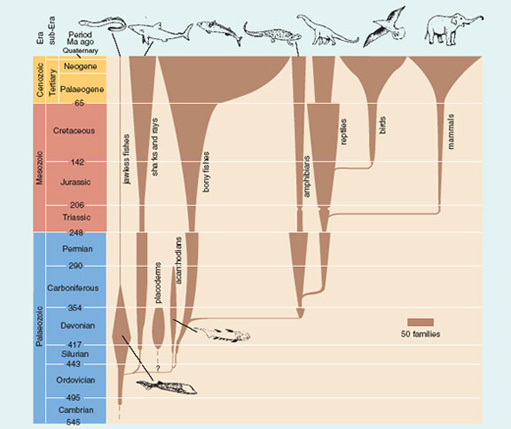
Which of today's five vertebrate classes (Section 1.3) were in existence by the end of the Triassic Period (see Figure 14)?
Answer
Fishes, amphibians, reptiles and mammals had all evolved by then, about 206 Ma ago.
SAQ 20
In terms of families, which living vertebrate groups shown in Figure 14 are at their highest diversity today? Which living groups were more diverse in the past, and when was their peak of diversity?
Answer
Sharks and rays, bony fishes, birds and mammals are at their maximum family diversity today. Jawless fish were most diverse in the Devonian; amphibians in the Permian; and reptiles in the Cretaceous.
In discussions such as this, it is important to be aware which taxonomic category (e.g. order, family or genus) is being considered because the picture can change if other measures of diversity (e.g. species) are used.
Activity 1
From Figure 14, estimate roughly (to the nearest five) the number of living families of reptiles and of mammals. Then, using the data on species numbers given above, work out which of these two groups has, on average, more species per family.
Answer
Figure 14 shows approximately 40 families of living reptiles. So the average number of species per family is 12 000 divided by 40 ≈300. There are about 150 families of living mammals and 6000 species, giving an average of 6000 divided by 150 ≈40. Thus living reptiles have far more species per family than mammals.
As we have already seen, the vertebrates were initially slow to diversify. From their Cambrian start, it was not until late Ordovician times that the early jawed fish evolved into different groups, though some were subsequently to become extinct (the placoderms, see Atlas, pp. 76-77, and acanthodians, see Atlas, p. 83). From Figure 14 it becomes clear why the Devonian is often called the 'Age of Fishes': not only was there a greater diversity of fish groups than at any other time but most of the other vertebrate classes had yet to evolve.
Figure 14 reveals a variety of patterns in fish evolution, including some major setbacks. The placoderms became extinct at the end of the Devonian, coincident with a decrease in early tetrapod diversity. Sharks, rays and bony fishes suffered a major extinction at the end of the Permian, coincident with a similar setback for amphibians and reptiles, part of a mass extinction event. Today, the total number of fish families is at its highest ever, mainly due to the bony fishes.
SAQ 21
When did the total number of bony fish families begin to increase steeply from some 95 families to their present total of about 260?
Answer
In early Tertiary (Palaeogene) times.
Let's now look at the history of tetrapods. After emerging in late Devonian times, tetrapod communities were initially dominated by amphibians, especially in Carboniferous and Permian times, when a wide variety of amphibian groups evolved and died out. Amphibians remained large during the Palaeozoic Era, often 1-2 m long - huge by the standards of today's frogs, toads and newts, which have a relatively recent, Mesozoic origin. The first reptiles appeared in the early Carboniferous.
SAQ 22
What crucial adaptation did reptiles evolve that freed them from the amphibian dependence on being near water to lay eggs and develop tadpoles?
Answer
A shelled egg that did not dry out in air (the amniotic egg) (Section 6.1).
At first the earliest Carboniferous reptiles were quite small, superficially lizard-like predators. They lived mostly on arthropods, such as dragonflies and cockroaches, in the dense equatorial Carboniferous forests and swamps. The energy of sunlight trapped during photosynthesis became stored in vast amounts of plant litter that accumulated on the floor of the forests and in swamps. Eventually, this debris was buried, compressed and converted by heat and pressure into coal; 300 Ma later the energy from Carboniferous sunlight fuelled the Industrial Revolution.
Not until Permian times did reptiles begin to diversify widely and increase in size; some even returned to living in the sea. Others evolved certain mammal-like features, such as the beginnings of tooth differentiation. This allowed for separate functions such as large dagger-shaped teeth for defence and killing prey, and smaller cheek teeth for efficient processing of food before digestion. One of these groups that survived the end Permian mass extinction eventually evolved into the first mammals in the late Triassic.
Activity 2
Using Figure 14, measure the approximate number of families (to the nearest five) present in the four main vertebrate groups (sharks and rays, bony fishes, amphibians and reptiles), first at the end of the Permian and then in the early Triassic. From these totals, calculate the percentage change in family diversity.
Answer
The approximate numbers of end Permian families are: sharks and rays (20), bony fishes (25), amphibians (35) and reptiles (20) - total 100. Early Triassic families: sharks and rays (10), bony fishes (10), amphibians (10) and reptiles (5) - total 35. There was therefore a 65 per cent decrease in family diversity across the Permian-Triassic boundary.
After the end Permian extinction event, the reptiles recovered and gradually became the dominant group by the end of Triassic times, though both reptiles and amphibians were set back by another, late Triassic, extinction event.
SAQ 23
Which group do you think was mainly responsible for the dominance of reptiles in the Mesozoic?
Answer
It was, of course, the dinosaurs, though dinosaurs were only one of many kinds of Mesozoic reptiles.
Dinosaurs were a special group of reptiles - special not least because, after their origin in the late Triassic, they became the dominant land animals for over 150 Ma. They filled almost every niche possible for large land vertebrates. They included carnivores, herbivores and a few omnivores (mixed diet), and ranged in size from that of a chicken to vast plant eaters, such as the Brachiosaurus, which grew up to 25 m long and weighed up to 40 tonnes. Dinosaurs never, however, took to the air or to the oceans: other large reptiles - the pterosaurs - dominated the skies, and marine reptiles such as ichthyosaurs, plesiosaurs and mosasaurs flourished in the sea. The major surviving groups of reptiles, the crocodiles, turtles and tortoises, lizards and snakes also all appeared in Mesozoic times. Much evidence suggests that birds evolved from dinosaurs in late Jurassic times.
The great success of the Mesozoic reptiles came to a dramatic halt at the end of the Cretaceous. Reptile diversity was cut back from about 60 families to 30 and never fully recovered (Figure 14). The bony fishes rapidly increased in diversity in the early Tertiary.
For a long time after their late Triassic origin, mammals remained small, perhaps nocturnal, shrew-like creatures living in the nooks and crannies of the dinosaur world. But once the large Mesozoic reptiles had become extinct, mammals were able to diversify into the wide range of niches that became vacant. Plants, which also suffered some extinctions at the end of the Cretaceous, continued to have a role in the evolution of both vertebrates and invertebrates. The rise of flowering plants (angiosperms) and pollinating insects, together with climate change in the early Tertiary, promoted the evolution of grasses, which in turn supported the spectacular rise of grazing mammals. It was perhaps the climate-driven change in vegetation that promoted our own evolution from tree-dwelling primates in late Cenozoic times. Global cooling as part of the initial descent into the Quaternary Ice Age caused retreat and fragmentation of the extensive forests of central Africa and their replacement by more open grasslands. This gave an adaptive advantage to a group of more upright-walking primates - the hominids. Our own species, Homo sapiens, appeared only 100 000 to 150 000 years ago (depending on how the origin of the species is defined).
Conclusion
This free course provided an introduction to studying Environment & Development. It took you through a series of exercises designed to develop your approach to study and learning at a distance, and helped to improve your confidence as an independent learner.
Acknowledgements
The content acknowledged below is Proprietary (see terms and conditions). This content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence
The author of this course is Peter Sheldon.
Course image: Ryan Somma in Flickr made available under Creative Commons Attribution-NonCommercial-ShareAlike 2.0 Licence.
Grateful acknowledgement is made to the following sources for permission to reproduce material in this course:
Figures 1 and 2 Peter Sheldon;
Figure 3a National Museum of Wales;
Figure 3b Courtesy of Jason Dunlop;
Figures 5-12 Peter Sheldon;
Figure 13 John Watson;
Figure 14 Adapted from Benton, M.J. (2000) Vertebrate Palaeontology, second edition, Blackwell Science.
John Watson
The content acknowledged below appears in pages 58-83 of 'The Atlas of the Prehistoric World' by Douglas Palmer published by Marshall Editions, London (which forms part of The Open University course S193 Life in the Palaeozoic) and grateful thanks are extended to them and to all rights-owners for their permission.
Thanks are also credited in the original publication for their kind help during production to: Dr Alan Smith, Department of Earth Sciences, University of Cambridge and Dr Norman Macleod, Natural History Museum, London.
58l, 58r, 59t, 59b, 62t, 63t, 64, 65l, 66b Simon Conway-Morris, University of Cambridge; 60, 61 Dr Peter Grimes; 62b Royal Ontario Museum; 63b, 65tr, 65br Dr Derek E.G. Briggs University of Bristol; 66t Gary Bell/Planet Earth; 67 NHPA; 68t William Campbell/DRK Photo; 68b Jens Rydell/Bruce Coleman; 69t, 72b Martin Land/Science Photo Library; 70 Richard Aldridge, University of Leicester; 71t BD/IPR/19-7 British Geological Society © NERC. All rights reserved; 71b, 73t, 73b, 76b, 77b The Natural History Museum, London; 72t Manfred Schauer; 74l, 75r, 80b Sinclair Stammers/Science Photo Library; 74r Department of Earth Sciences, Cardiff University, Wales; 75l Kaj R Svensson/ Science Photo Library; 76t Breck P Kent/Oxford Scientific Films; 77t Eric and David Hosking/Corbis; 78 Zig Leszczynski/Oxford Scientific Films; 79, 82r University Museum of Zoology, Cambridge; 80t G I Bernard/NHPA; 81t Sarah Finney; 81b The Manchester Museum, The University of Manchester; 82l Andrew Mounter/Planet Earth Pictures; 83 National Museums of Scotland.
60/61, 74/75, Jim Channell; 64/65, 82/83 Colin Newman; 78/79, Robin Bouttell, Marshall Editions Development Limited
Three-dimensional maps produced by Lovell Johns Limited, Witney, using illustration by Hardlines Limited, Charlbury and based on data supplied by Paleomap Services Limited, Cambridge.
Two-dimensional maps by Eugene Fleury, Marshall Editions Development Limted
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
Copyright © 2018 The Open University