Surviving the winter
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Tuesday, 23 April 2024, 8:56 AM
Surviving the winter
Introduction
In this course, we study one aspect of the fluctuating nature of an organism's environment. We consider how organisms living in a temperate climate, such as that in Britain, are adapted to cope with winter. You will see that there is much diversity of adaptations among organisms, with different species coping with the demands of a fluctuating environment in quite different ways. As cyclic variations are a widespread feature of environments, the range of adaptations to them is an important source of biological diversity.
This OpenLearn course provides a sample of level 1 study in Environment & Development
Learning outcomes
After studying this course, you should be able to:
identify the four main strategies shown by organisms for coping with winter
appreciate and give examples of the levels and types of explanation used for understanding these strategies
describe ways in which the strategies can be subjected to experimental manipulation
provide examples of how plants, birds and mammals can remain active through winter
give examples of organisms that survive the winter as juveniles.
1 Surviving the winter
1.1 Living in a fluctuating environment
The great majority of organisms live in environments that fluctuate extensively and cyclically. For example, an animal such as a limpet living on a sea-shore experiences a 12-hour cycle in which it is alternately exposed, first to the air, the wind and the heat of the Sun, then to pounding by the sea. Life in the natural world exposes organisms to a diversity of environmental cycles, acting on a variety of time-scales. The most obvious cycles are night and day, with a period of 24 hours, and winter and summer or wet and dry seasons over a year; less obvious, but important for many organisms, is the lunar cycle (28 days). Even organisms that live deep in the oceans or under the ground may be exposed to cyclic variations in their environment.
1.1.1 A diversity of strategies
Faced with an environment that becomes relatively hostile with the onset of winter, an adult organism can, broadly speaking, do one of four things:
It can maintain an active lifestyle, adapting in various ways to the prevailing conditions. The robin (Erithacus rubecula) is an example of such a species; so are evergreen trees.
It can abandon an active lifestyle, adopting an inactive existence for the duration of winter. The hedgehog (Erinaceus europaeus) spends the winter in hibernation. Many plants remain dormant below ground during the winter.
It can die before the onset of winter, as many adult insects do, but their offspring can survive the winter, for example as an egg or a pupa. Some plants survive the winter as seeds.
It can leave, migrating to a part of the world where conditions are favourable during the winter months. Each year, swallows (Hirundo rustica) leave Britain in autumn and migrate to Africa, whilst other birds such as barnacle and pink-footed geese migrate to Britain from further north.
Each of these four strategies is more applicable to some kinds of organisms than others. Long-distance migration, for example, is only an option for species that can store energy or can feed continuously (e.g. reindeer, Rangifer tarandus). For each of the three groups of species in Table 1.1 there is one strategy that is not an option. Later in this course, we will focus on each strategy in turn, examining how it is manifested in different groups of organisms. Table 1.1 summarises these alternative strategies and gives some examples of organisms that use each of them. This table provides a conceptual framework for much of the course.
| Strategy | Plants | Insects | Vertebrates |
|---|---|---|---|
| 1 'Tough it out' | |||
| Maintain an active adult existence by altering behaviour and physiology with changing conditions. | Plants that continue to photosynthesise, e.g. evergreen trees. | Not an option for most insects. | Mammals that remain active, e.g. fox. Birds that remain active, e.g. robin. |
| 2 'Opt out' | |||
| Maintain an inactive existence as adults for the duration of the hostile period. | Perennial plants that survive the winter by dying down or going dormant above-ground and/or forming subterranean storage organs, e.g. bulbs and rhizomes. | Survive the hostile period in a state of torpor, e.g. certain wasps, bees and butterflies. | True hibernation in small mammals. Not an option for most birds. Winter torpor in amphibians and reptiles. Freeze-tolerance in amphibians. |
| 3 Juvenile survival | |||
| Survive the hostile period as some non-adult phase of the life cycle. | Survive the hostile period only in the form of seeds. | Survive the hostile period in the form of eggs, larvae or pupae. | Not an option for vertebrates |
| 4 'Go away' | |||
| Migrate as adults to a location where conditions are favourable. | Not an option for plants. | Some butterflies migrate north each spring and summer. | Migration in some birds and mammals. |
Each of the four strategies can be discussed at different levels of explanation. For example, with migration of birds we can consider the molecular and cellular processes involved in energy storage and its utilisation for long-distance flight. We can discuss the physiological and structural features of birds' wings that allow long distance flight. Alternatively, we can consider migration in an evolutionary context, discussing the selection pressures that favour migration. This approach leads us into the ideas of types of explanation. We will see firstly that organisms carry out certain activities, such as breeding, at those times of year when it is most adaptive for them to do so (i.e. to maximise their fitness) and, secondly, that those activities are performed in response to appropriate environmental cues. For example, many animals whose food is available mainly in summer ensure their survival in winter by building up fat reserves in the autumn.
It is important to separate arguments about adaptation during evolution, referred to as ultimate types of explanation, from the analysis of causal mechanisms, referred to as proximate types of explanation. To this end, a distinction is made between proximate and ultimate factors. Ultimate factors are those features of the environment that have favoured the evolution of particular adaptations; for example, many small mammals have evolved hibernation as an adaptation for coping with adverse winter conditions. Proximate factors are those specific features of the environment that elicit specific responses by organisms; long nights elicit a variety of responses in many small mammals, such as increased foraging, laying down fat and hoarding food. Thus proximate factors may operate at several levels. We will see that uniformity and diversity can only be fully understood with reference to both types of factors (ultimate and proximate) as well as several different levels of explanation.
1.2 Response to winter: understanding at different levels
Winter in a temperate region poses a number of environmental problems for organisms. Most obviously, average temperatures are lower than at other times of year and there are frequent frosts. Frost is highly significant for living organisms because water forms such a large proportion of their body tissues; for the great majority of organisms, freezing of their tissues leads to death. Secondly, because, as shown in Table 1.1, many adult organisms die, go into hiding or migrate in winter, many of those animals that remain have a greatly reduced supply of food (i.e. plants for herbivores and prey for carnivores). A third significant factor in winter is that the daylight period becomes markedly shorter than at other times of the year. For plants that require light for photosynthesis and for animals that use daylight vision to find food, shorter days in winter severely restrict their ability to acquire the energy and nutrients that they need to support life. Finally, a major problem for plants in winter is shortage of water, which arises for two reasons. Firstly, when water becomes frozen in the ground, it is no longer available for plants to take up through their roots. Secondly, low temperatures greatly reduce the capacity of plant roots to take up water that is available, because their physiological processes are slowed down and, often, the transport tubes (xylem) become blocked by air bubbles, so are unable to function. Here we will introduce these problems at a number of levels: Sections 1.2.1-1.2.3 invoke proximate types of explanation whilst Section 1.2.4 considers ultimate types of explanation.
1.2.1 The molecular level
It is common knowledge that the freezing point of pure water is 0°C. Often, however, the temperature of water can fall below 0°C without it freezing, for two reasons:
Any solvent containing a dissolved substance has a lower freezing point than when pure, which is why the sea freezes at a lower temperature than clean freshwater.
The occurrence of supercooling, the phenomenon by which a fluid remains liquid at a temperature below its normal freezing point. Freezing occurs when water molecules become aligned in a particular pattern that leads to the formation of ice crystals. It begins when two molecules become aligned, a process called nucleation, and ice grows from a nucleation point. Whether or not water molecules become appropriately aligned depends on the conditions, so the temperature at which freezing occurs varies within a narrow range. Supercooled water freezes abruptly if an ice crystal is added to it. Small objects such as microbes also act as nucleation points, including certain bacteria on the surfaces of plant leaves. For example, Pseudomonas syringae cells act as ice-nucleation points, causing ice to start forming at −1°C. In the absence of these bacteria, ice does not form on plants until about −5°C. Genetically engineered strains of P. syringae, called 'ice-minus', have been produced which lack ice-nucleating characteristics. When these bacteria are sprayed onto the leaves of crop plants, they make them more frost-resistant.
Fishes living in temperate and arctic habitats remain active through the winter (strategy 1 in Table 1.1), swimming about in very cold water. The winter flounder (Pseudopleuronectes americanus), for example, swims actively in water at a temperature well below the freezing point of its blood. What prevents its tissues from freezing? Two hypotheses, both of which invoke molecular levels of explanation, have been proposed. The ice-prevention hypothesis is that the tissues of fishes contain compounds that act as antifreeze, lowering the overall freezing point of their cells and body fluids. A number of such compounds have been identified, but they do not occur in some body fluids, such as urine and the fluid in the eye, suggesting that freezing is only prevented in some parts of the body. The alternative hypothesis, proposed by Valerio et al. (1992) is that ice is excluded from the body by a surface barrier. The skin of the winter flounder contains peptides (short chains of amino acids) which prevent ice formation. These molecules act in two ways: by lowering the freezing point of water in the skin and by binding to water molecules and so preventing them from binding to each other to form ice crystals. As described above, ice grows from nucleation points and the skin prevents ice formation at the fish's interface with very cold water.
1.2.2 The cellular level
The water in plant and animal tissues has two major components: the intracellular fluid (within cells) and the extracellular fluid, which fills the spaces between cells. When a tissue freezes, ice typically forms first in the extracellular fluid. Ice formation has two harmful effects:
It disrupts cell walls and cellular membranes.
The formation of ice in extracellular fluid effectively removes water from solution, thereby increasing its solute concentration. The gradient in solute concentration between the extracellular fluid and the cells it surrounds causes water to move out of the cells into the extracellular spaces (a process called osmosis), so that the cells collapse.
The destructive effect of frost on plants is a familiar consequence of these forms of cellular dysfunction. Frosted foliage collapses and turns brown, a result of the destruction of tissues in which ice has formed. These effects are especially obvious in some non-native plants, which are not adapted to survive frost.
1.2.3 The physiological and behavioural levels
Anticipation of winter
If organisms are to survive winter, they must be well prepared for cold weather and sometimes for reduced supply of food. Physiological changes such as shedding leaves and building up fat reserves, and behaviours such as hoarding food, must be completed before winter begins. In order to be prepared, organisms need to anticipate the onset of winter, which is done in two ways, each of which has its own associated physiological mechanisms. First, they could respond to environmental cues that predict that winter is imminent, e.g. lower temperatures and shorter day lengths.
Question 1
Which of these cues is the more reliable?
Answer
Changes in day length, which follow an identical pattern every year. We know from personal experience that temperature is very variable and that cold weather comes earlier in some years than others.
Secondly, animals could have an internal clock which tells them what time of year it is, just as a calendar tells us the date.
Photoperiodism
A great deal of research has been carried out into the way that organisms respond to changes in day length, i.e. the relative durations of light and darkness in a 24-hour period. This relationship is expressed as the ratio of the number of hours of light (L) to the number of hours of darkness (D), i.e. L : D. Photoperiodism is defined as the responses of organisms to changes in the L : D ratio. For example, flowering plants can be divided into three categories on the basis of the effect of photoperiodism on the onset of flowering:
Short-day plants flower in early spring (e.g. primrose, Primula vulgaris) or in the autumn (e.g. Chrysanthemum spp.), when L is small relative to D.
Long-day plants flower in the summer (e.g. potato, Solanum tuberosum, or lettuce, Lactuca sativa), when L is large relative to D.
Day-neutral plants are not affected, in terms of flowering, by changes in the L : D ratio (e.g. groundsel, Senecio vulgaris).
Horticulturalists have long been familiar with these effects and exploit them to get plants to flower at times that suit them. If you go to the Chelsea Flower Show, which takes place in May, you see spring, summer and autumn plants, raised under artificial conditions, all in bloom at the same time.
Question 2
How can chrysanthemums be made to flower later in the year than normal?
Answer
By keeping them under high L : D conditions during the spring, using artificial lights, and transferring them to low L : D conditions just before they are required to bloom.
Simply observing that primroses, for example, flower in early spring is consistent with the hypothesis that they flower in response to short light periods, but it does not preclude alternative hypotheses, such as that they flower in response to increased temperature. However, primroses kept indoors under consistently long light periods and a variety of temperatures do not flower, confirming that onset of flowering is indeed a response to short light periods. Photoperiodism can only be revealed by means of experimental manipulations in which plants are kept under different L : D regimes, and in which other environmental cues to which they might respond are held constant. Variations on this kind of experiment tell us other things about photoperiodism. For example, some plants flower in response to a single exposure to an appropriate L : D ratio. Others flower only if an appropriate L : D ratio is sustained for several days. A pigment, called phytochrome, which switches between 'dark' and 'light' forms, is involved in sensing the length of the light and dark periods.
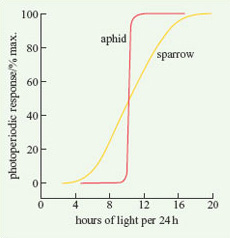
For a specific physiological response, there is typically a particular L : D ratio at which the response starts to occur. The critical photoperiod is defined as that L : D ratio at which 50% of the population being studied switches from one state to another. There is considerable variation in the preciseness of a particular organism's response to a critical photoperiod. Figure 1 shows two examples of critical photoperiods for animals. Whereas all aphids switch from sexual to asexual reproduction at an L : D ratio of 10 : 14, male sparrows start to show testicular development at an L : D ratio of about 8 : 16, but all do not mature unless the ratio is about 16 : 8.
In some animals, the physiological basis of this effect is known. The hormone melatonin is secreted by the brain during the dark period, with the result that in winter blood levels of melatonin are much higher.
Circannual Clocks
The physiology and behaviour of microbes, plants and animals show cyclical changes on a diversity of time-scales. Most familiar to humans are circadian rhythms, which determine patterns of sleep and wakefulness and of changes in body temperature over a 24-hour period. There is also evidence that certain annual rhythms are controlled by internal clock mechanisms, called circannual clocks (circannual means 'about a year').
Whatever the time-scale over which they operate, the existence of endogenous clocks can only be determined by experimental investigation. Simply observing that an animal or a plant shows a daily or an annual cycle of activity or physiology does not preclude the possibility that it is responding to rhythmic changes in the environment. The crucial experiment is called a free-running experiment, in which organisms are kept under conditions in which they are unable to detect normal cycles in external features of their environment. For example, captive alpine marmots (Marmota marmota, a member of the squirrel family) have been kept for long periods under constant temperatures and constant L : D ratios. Despite having no known cues that winter was approaching, they showed a 100% increase in food intake in the autumn, just as they do in nature, in preparation for hibernation. Kenagy (1981) found that chipmunks (Eutamias minimus and E. amoenus, also in the squirrel family) kept under combinations of three photoperiods (L : D ratios 8 : 16, 12 : 12 and 16 : 8) and two temperatures (5 and 23°C) maintained normal cyclical patterns of testis growth, body mass, water consumption, locomotion and winter torpor.
Linkage of animal reproductive cycles to winter
The reproductive success of organisms is crucially determined by the time of year when they breed. Many birds living in Britain breed in the spring, with the result that they are able to feed their young at that time of year when there is most suitable food available. For many kinds of animal, all parts of the reproductive process, from mating to birth, follow the end of the winter. A complication for some larger mammals, however, is that there is a long gestation period (the interval between conception and birth, nine months in humans). Along gestation period and giving birth early in the spring are not easily reconciled with being inactive in winter. Figure 2 shows how the reproductive cycles of four mammals native to Britain are related to the winter.
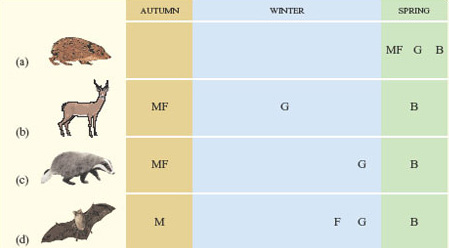
The hedgehog (Erinaceus europaeus) has a short reproductive cycle and so can complete the entire process in the spring, mating soon after emerging from hibernation. The red deer (Cervus elaphus), with a long gestation period, mates in the autumn (the rut) and gives birth in spring, remaining active through the winter. The other two species shown in Figure 2 have gestation periods that are too long for the entire reproductive cycle to be completed in the spring, but too short to occupy the whole winter. Badgers (Meles meles) mate in the autumn and the eggs are fertilised immediately. Implantation of the zygote into the wall of the uterus is, however, delayed for several months, during which the female spends short cold spells in a state of torpor in an underground den. The noctule bat (Nyctalus noctula) mates in the autumn but the eggs are not fertilised. Instead, females store sperm in their reproductive tract until late winter, when fertilisation occurs and gestation begins. Throughout this time, they are hibernating.
These different reproductive patterns are controlled by environmental cues in much the same way as the flowering of plants. Red deer, badgers and bats are called short-day breeders; the development of their gonads and their sexual behaviour is stimulated by the lengthening dark period characteristic of autumn. Hedgehogs and many other small mammals are long-day breeders; their gonad development and sexual behaviour are triggered by a decrease in the dark period.
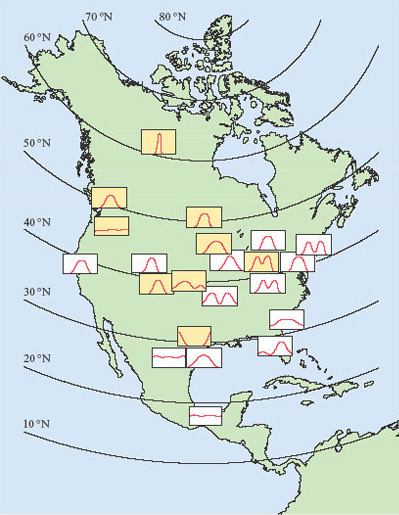
Figure 2 illustrates how diverse are the reproductive cycles of a single group of animals, the mammals. Deer, badgers and hedgehogs are very different animals, however, and before we leave this brief account of seasonal breeding, it is important to emphasise that there is enormous diversity, even among closely related species. Figure 3 shows the breeding seasons of members of a single genus, Peromyscus, in North America. Peromyscus is a genus of small American rodents that includes a variety of mice, such as the deermouse, white-footed mouse and cactus mouse.
Question 3
How does the breeding season in Peromyscus species change with latitude?
Answer
The breeding season of Peromyscus is limited to a single, three-month period in northerly latitudes, but is continuous through the year in Mexico. In between, there is considerable variation in the duration of the breeding season; in some regions at intermediate latitudes, there are two peaks in breeding activity.
Winter fat reserves and reproduction
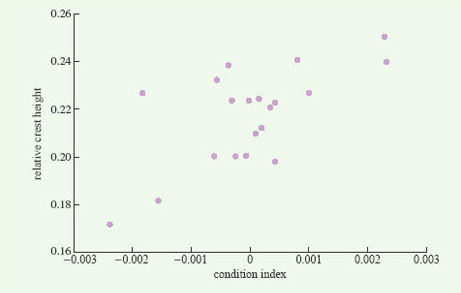
For some animals in which breeding begins as soon as the winter has finished, energy reserves accumulated in the previous autumn may be important, not only for winter survival but also for reproductive success in the following spring. In newts, for example, as in other temperate amphibians, nutrient reserves are built up in the late summer and autumn, in both the liver and the abdominal fat stores. These reserves are only partly used up in the winter, when newts are largely torpid underground, and the remainder plays a key role in reproduction. Female newts use their fat reserves to produce yolk for their eggs; males use theirs to develop a large dorsal crest, which is crucial in mating, females responding positively only to males with large crests (Figure 4). A population of great crested newts (Triturus cristatus) on the Open University campus has been studied in detail by John Baker. He weighed and measured males as they migrated towards their breeding pond in early spring and, for each male, he calculated a 'condition index'. Males in good condition (i.e. with a condition index value greater than zero) were heavy for their length, because of their larger fat reserves. Later in the year, Baker recaptured the same individuals, now in the pond, and measured their dorsal crest, which develops after newts have entered the water. He found a positive correlation between their crest height and their condition index measured a few weeks earlier (Figure 5). Thus, a male newt's attractiveness to females, and hence his reproductive success, is partly determined by the amount of fat that he has left over after hibernating during the winter.
1.2.4 The evolutionary level
Life histories and trade-offs
In this section, the emphasis switches from proximate (molecular, cellular, physiological and behavioural) types of explanation to ultimate types of explanation. In order to proceed, we need to understand two key concepts: life history and trade-off. Both of these concepts are important tools in organising thoughts about why organisms are so diverse. An organism's life history is the set of key biological events in its life, including birth, growth, reproduction, sometimes migration, and death. Life histories are distinguished from life cycles, which are a detailed description of the sequence of stages in an organism's life. Life histories can be subjected to quantitative analysis. A familiar example of a life history is that of an annual plant which, following birth (germination), grows, flowers (reproduces), sets many seed and then dies. A contrasting life history is that of large-bodied mammals such as African elephants or blue whales. Following birth, these animals grow to a very large size before giving birth to (usually) one offspring at a time. There is then a period of recovery before new young can be produced. Death may follow 20-30 years after the first birth, during which time perhaps 8-10 young may have been born.
Between the extremes of an elephant and an annual plant lie the life histories of many species. If these life histories are subjected to quantitative analysis, for example by plotting fecundity (number of offspring) against chances of survival, the results indicate that trade-offs are at work. For instance, organisms with a high fecundity have a lower chance of future survival. What are the reasons for these results? The patterns between species reflect processes that have occurred within species and can be given proximate explanations. In proximate terms, each behavioural, physiological or biochemical activity has an energetic cost. For a given energy input (food or light intake) an organism cannot afford to indulge in many different costly activities. Therefore trade-offs have to occur. Let us take the example of a short-lived plant in which there is genetic variation in the amount of photosynthetic product (e.g. starch) that can be stored in the root. Some individuals can store more in the roots than others. Those individuals that store less in the roots divert the energy into seed production. The probability of plants surviving after flowering is inversely related to the amount of seed produced. Thus plants that put less stores into roots have a higher probability of dying after flowering, in contrast to plants that store more in the roots. The latter produce fewer or smaller seeds and hence have a higher probability of survival after flowering.
Natural selection can operate on this genetic variation. It may be that under certain environmental conditions the storers are favoured, and under other environmental conditions, those that produce many seeds are favoured. It may also be that both strategies are favoured by the same environmental conditions. Thus both seed producers and storers do well in winter, i.e. there are two equally adaptive solutions to the same environmental problem. Hence we can see how genetic variation underpinning life history variation due to trade-offs in individuals can, through natural selection and subsequent speciation, be represented as life history variation between species. In conclusion, it is possible to move from proximate explanations of trade-offs and life history variation in individuals, to ultimate explanations of life history variation between species. This course will cover many examples of trade-offs, which act as important constraints on organism structure.
Plant life histories
To review ideas on life histories, let us consider some plant examples. Among plants, there are three broad categories of life history, each of which has different implications for how they respond to the onset of winter:
Annuals As described above, these plants complete their life cycle in a single year. Most annuals in temperate regions set seed during the summer and then die before winter (Table 1.1, strategy 3). They represent the extreme version of the high seed-producers discussed above.
Biennials or short-lived herbaceous perennials These plants have the capacity for storage of photosynthetic product(s). In this particular category are plants that live for two or a few years, flowering and setting seed only in the second or final year. Since biennials that did not survive their first winter would fail to reproduce, they must be adapted for winter survival. They allocate some of their resources to growth in the first year, and some to accumulating stored reserves in their roots. Their foliage may die back with the onset of winter (strategy 2) but their stored nutrients and energy enable them to grow quickly in the second year, prior to flowering. Thus, whereas growth (vegetative) and reproductive phases of the life history occur in the same year in annuals, they are separated into different years in biennials.
Long-lived perennials These plants may persist for many years, typically reproducing many times. Perennial plants include herbaceous plants that die back each year (strategy 2), and woody plants, which possess a number of adaptations for surviving the winter (strategies 1 and 2). There may be a long juvenile period before the first reproduction event.
For smaller biennials and perennials, the typical overwintering strategy is for those parts of the plant above ground to die back, leaving an underground storage organ, such as a tap root, a bulb or a rhizome, to survive the winter. For larger plants such as trees, this option is not viable, because reconstructing the above-ground parts of the plant every year would preclude any long-term growth. Trees have two main strategies for surviving the winter, belonging to either the deciduous or the evergreen category (strategies 2 and 1 respectively). Deciduous trees are those that shed all their leaves in a particular season, usually the autumn. Evergreen trees retain leaves all year round; they do drop and replace their leaves, but only some at a time. While trees do not possess discrete storage organs, they store energy-releasing compounds and other nutrients during the winter, in their trunks and roots.
Storage during the winter involves risks because, among temperate animals, there are herbivores that follow strategy 1, remaining active through the winter. Plant storage organs are a vital food source for such animals and many individual plants fail to survive the winter because their storage materials have been consumed by animals or fungi.
We will now discuss in more detail the means by which selected groups of organisms survive the winter, with reference to the four strategies in Table 1.1. Throughout the discussion of these strategies, we will move between different types and levels of explanation. It will also be clear that certain strategies are only open to certain taxonomic groups.
1.2.5 Summary of Sections 1.1 and 1.2
The majority of organisms are exposed to environmental fluctuations, including seasonal change in climate. In this course, we focus on the effects of winter.
Organisms have evolved a range of strategies to cope with winter. Thus this common environmental variable has led to a diversity of responses.
The strategies for coping with winter can be considered with respect to different levels and types of explanation.
Molecular and cellular level responses to winter include the prevention of freezing through the production of antifreeze molecules such as peptides.
Physiological and behavioural responses include detecting the onset of winter through changes in the L : D ratio which prompts alteration of sexual behaviour. The reproductive cycles of many organisms are linked to the L : D ratio. Many of these effects can be investigated by experiment.
The life histories of organisms can be viewed as the products of trade-offs in biological processes.
1.3 Strategy 1: Remaining active through the winter ('tough it out')
1.3.1 Evergreen plants
In temperate regions, the most prominent evergreen plants are coniferous trees, or conifers (phylum Coniferophyta). Conifers dominate large portions of the Earth's land area, particularly at northern latitudes and high altitudes. This distribution reflects their ability to withstand long periods of cold weather. The major problem faced by conifers in winter is lack of water. Water that has turned into ice is not available to plants and, at freezing temperatures, plant roots are able to absorb such water as is available only very slowly. If conifers are not to die of desiccation they must, therefore, reduce the rate at which they lose water. The needle-like shape of conifer leaves reduces the rate at which water is lost from their surfaces and so reduces a tree's requirement for water.
Though very small compared to the leaves of most deciduous trees, pine needles are relatively thick, in comparison to the broad, flat leaves of deciduous trees. Consequently, their surface area is small relative to their volume, reducing water loss. Evaporation is further reduced by a thick, waxy cuticle that forms the outer surface of needles and by the stomata (pores through which leaves exchange gases with the air) being positioned in sunken pits.
Not all conifers are evergreens, and not all evergreens are conifers. There are some ten species of larches (genus Larix) that live mostly at high altitude in the Northern Hemisphere; all are deciduous, dropping their needles at the onset of winter. The holm oak (Quercus ilex), also known as the evergreen oak, is not a conifer, but retains its leaves through the winter. The holm oak is a native of continental Europe, from the Mediterranean to Brittany, that has been introduced into Britain.
As explained in Section 1.2.4, life histories involve trade-offs between many factors and this principle is well illustrated by a comparison between deciduous and evergreen trees. By retaining their leaves through the winter, evergreens do not bear the cost, as deciduous trees do, of reconstructing their entire photosynthetic apparatus each spring. However, the adaptations that enable their leaves to survive the winter make them less efficient in the spring and summer than those of deciduous trees. Another trade-off is that conifers have a simpler system of water-conducting cells which is less efficient when water is plentiful, but better (because it is less likely to block) when water is scarce and freezing occurs.
1.3.2 Birds and mammals
Birds and mammals are endotherms, meaning that they produce and retain a lot of heat within their own tissues, rather than absorb heat from their environment, as ectotherms, such as insects and reptiles, do. The terms endotherm and endothermy are now often used in preference to homeotherm and homeothermy, which refer to the ability of birds and mammals to maintain a more or less constant body temperature. Some endotherms, as you will see later, do not maintain a high body temperature at all times, and some ectotherms, such as larger reptiles, maintain a constant body temperature for long periods, even though the temperature of their environment changes.
Whilst endothermy allows some birds and mammals to remain active during winter, it also places formidable demands on those animals. Reduced environmental temperatures increase the amount of heat that they need to generate internally to maintain a constant temperature, at a time when the amount of food available to them is much reduced. For birds and many mammals, shorter day length in winter reduces the time available for finding food. It has been estimated that a small bird, such as a great tit, which feeds on seeds and small insects, has to find food items at an average rate of one every ten seconds during daylight hours through the winter. The extreme challenge posed to birds by the shortage of food in winter is illustrated by the impact of bird-tables in urban areas, which supplement their natural diet. Studies by the RSPB (Royal Society for the Protection of Birds) suggest that, in urban areas of Britain, the provision of food at bird-tables significantly increases the winter survival rate of several garden bird species.
Maintaining body heat
Mammals that remain active in winter maintain a core body temperature of around 37-38°C; that of most birds is a little higher. In temperate habitats, thermal constancy is achieved despite the temperature of the environment varying between −20 and +20°C. For animals in polar regions, the problem is even more severe, their environment varying between −60 and +20°C. There are three principal ways in which endotherms can respond adaptively to cold conditions:
They can raise their metabolic rate so as to produce more heat to offset the increased heat loss.
They can improve external insulation between their body and the external environment, so as to reduce the rate of heat loss.
They can alter the pattern of blood circulation around their body so as to minimise the extent to which warm blood comes close to the skin (i.e. another form of insulation).
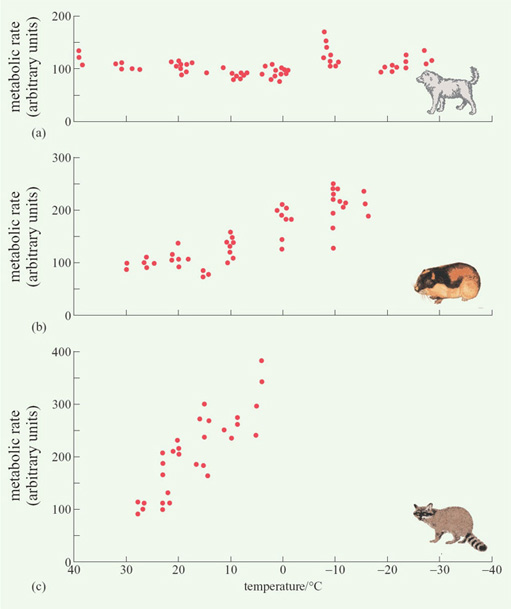
Considering the first of these processes, Figure 6 shows data from experiments in which three mammalian species, two from the Arctic and one from the tropics, were exposed to declining temperatures.
Question 4
In what way does the response to reduced temperature of (a) the Eskimo dog and (b) the arctic lemming differ from that of the tropical raccoon?
Answer
(a) The metabolic rate of the Eskimo dog shows no clear pattern of change (except possibly increasing at very low temperatures), whereas that of the raccoon increases almost linearly as the temperature decreases from 28 to 5°C. (b) The metabolic rate of the arctic lemming begins increasing at about 15°C, but more gradually than that of the tropical raccoon.
Mammals and birds living at high latitudes generally rely on processes 2 and 3, rather than 1, though, as we have seen from Figure 6(b), there are arctic species, such as the lemming, in which process 1 does play a major role.
Maintaining a high metabolic rate through a long winter requires that mammals and birds either maintain a high rate of food intake, or carry substantial energy reserves within their body, or show some combination of the two. They must also reduce heat loss during the winter. Many species maintain larger energy reserves, in the form of adipose tissue. The adipose tissue beneath the skin (subcutaneous fat) is also widely assumed to act as a thermal insulation layer, but this theory is erroneous. Adipose tissue is not much better an insulator than muscle. Only for marine mammals, such as seals, in which the adipose tissue forms a thick layer of blubber, is its role in thermal insulation significant. For birds and mammals, insulation is provided by feathers and fur respectively, which trap a layer of air next to the skin. Static air is a very poor conductor of heat, so that air trapped in plumage or fur reduces heat flow between an animal's skin and the outside. Bird plumage is a remarkably effective insulator; it represents only 5-7% of a bird's mass and trapped air makes up 95% of its total volume. The texture and often the colour of birds' plumage and mammals' fur changes with the onset of winter, in comparison to the summer. This change is effected during moult, a seasonal process in which a bird changes all or many of its feathers and a mammal replaces its summer coat with a thicker winter coat, and the converse in spring.
In birds, the plumage has two major components: contour feathers, which form the exterior surface; and down, which lies beneath the contour feathers. Most feathers consist, in varying proportions, of pennaceous (fan-like) and downy components (see Figure 7). The large feathers in the wings and tail are wholly pennaceous; many other small feathers close to the skin are pure down. In winter, the number of down feathers is markedly increased. Only in a few species has the total number of a bird's feathers been counted; hummingbirds have about 940, whereas a swan has 25 000. In the house sparrow (Passer domesticus) there are 11.5% more feathers in winter than in summer. Each feather has an individual muscle which enables it to be lifted away from the body. This capacity enables birds to regulate heat loss by altering the thickness of the air layer trapped among the feathers.
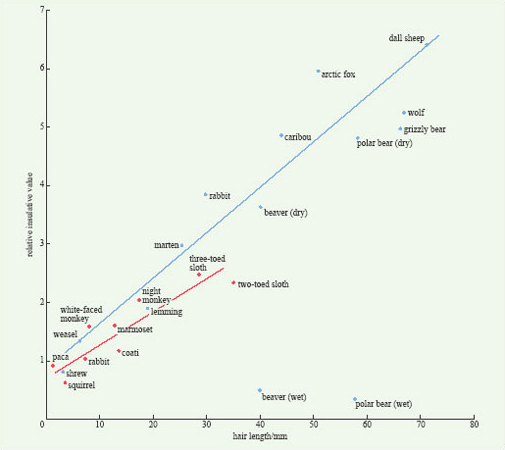
In mammals, fur contains fine hair close to the skin, underneath thicker and larger surface hairs. The insulative effect of hair is determined primarily by its length; the longer the hairs, the better is the coat as an insulator (Figure 8). Comparison of the insulative value of the pelts of arctic and tropical mammals with hair of similar length reveals that the pelts of arctic species are only slightly better insulators (Figure 8). The difference arises because arctic species have more hairs per unit area of skin. Figure 8 also shows how the insulative value of the coats of two species, the beaver and the polar bear, is completely eliminated when it becomes thoroughly wet.
In many species, the winter plumage or coat is very different in colour and pattern from the summer one. This seasonal change has nothing to do with temperature control, but is usually related to camouflage. The winter plumage of many birds, especially males, is much less brightly coloured than the summer plumage. In many polar mammals, such as the arctic fox (Alopex lagopus), the winter coat is white, making the animal well camouflaged in snow.
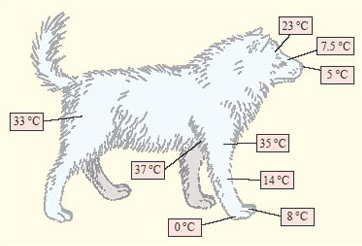
The third response to cold, varying blood flow to different parts of the body, is well illustrated by the Eskimo dog (Figure 9). At a temperature of −30°C the Eskimo dog maintains a core temperature of around +38°C, but adjustments in blood flow mean that the temperature of some parts of the body, notably the extremities such as the feet and the nose, is allowed to fall.
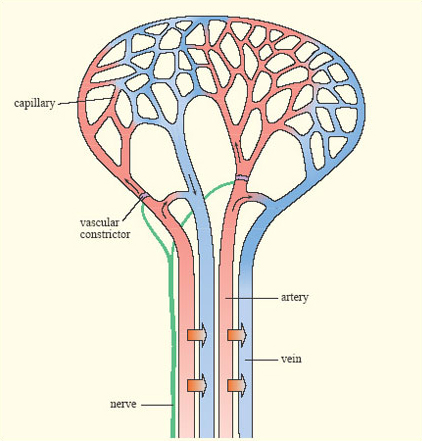
In many endotherms, cooling of the extremities is achieved by a heat exchange mechanism called a rete mirabile (pronounced 'reeta mirah-bilay' and meaning 'wonderful net', Figure 10). Warm blood passing in arteries towards the skin runs close to colder blood passing back in veins towards the body core. Thus warm blood passing outwards gives up much of its heat before it reaches the skin. This effect is enhanced by constriction of blood capillaries near the skin when it is cold.
Very small mammals and birds that remain active in winter face a problem. Because their surface area is relatively large in relation to their mass, they lose heat in cold conditions faster per unit mass than larger animals and the amount of extra fur or feathers they would need to insulate themselves would make them immobile. The dwarf hamsters (Phodopus spp.) of Siberia and Mongolia have adapted to this problem in some interesting ways. They remain active in winter but run around, feeding on seeds and vegetation, under the deep snow, where they are not exposed to the wind and the extremes of cold. Whereas larger animals typically build up their adipose tissue and put on weight as winter approaches, dwarf hamsters get markedly lighter and leaner. Males lose about 50% and females 30% of their body mass and adipose tissue falls from 35 to 5% of the total body mass.
Question 5
How might a lower body mass enable a dwarf hamster to better survive the winter?
Answer
Having a smaller body reduces an animal's energy requirement for locomotion.
Finally, consider the unfortunate pig. Pigs are large mammals and, as the result of selective breeding during domestication, they have so little hair that it provides no insulation against the cold. Pigs have the usual depots of subcutaneous fat found in other comparable mammals, but pig fat is no thicker than that of other mammals of similar size; indeed, domestic pigs have been selectively bred to be lean. The subcutaneous fat of pigs contributes little to their ability to survive cold weather. In cold conditions, the skin temperature of a pig falls much more than that of a human, due to restriction of the blood supply to the subcutaneous fat, so the poor hairless animal may feel cold in winter.
Storing food in winter
As described above, some animals solve the problem of remaining active in winter, when food is in short supply, by enlarging internal energy reserves in the adipose tissue. A problem with this strategy is that it increases body mass, which increases the energy cost of locomotion and, in extreme cases, may reduce mobility. An alternative strategy is to make external stores of food during the summer, when food is abundant, and to draw on them during the winter. Such behaviour, shown by a variety of birds and mammals, is called hoarding or caching (pronounced 'cashing').
There are two general kinds of hoarding behaviour. Larder-hoarding involves building up one or a small number of large food stores. Scatter-hoarding consists of hiding many individual food items in separate places over a wide area. An essential feature of a food cache, from the perspective of the animal that made it, is that it must not be exploited by other individuals of the same or different species. Typically, animals prevent theft by active defence in the case of larders, or by concealment if their caches are scattered. Scatter-hoarders include crows, nuthatches, tits, jays, squirrels and foxes; larder-hoarders include many small mammals, some birds, and honeybees.
Pikas (genus Ochotona) are herbivorous, guinea pig-size animals, belonging to the same mammalian order (Lagomorpha) as rabbits and hares, which live in high altitude and desert habitats of Asia and North America. They do not hibernate in winter but rely on cached food to supplement what they can find to eat beneath the snow. In late summer they gather vegetation, storing it as a large haystack in a crevice beneath a rock, which they defend against their neighbours. Laboratory experiments on a variety of mammals reveal that hoarding behaviour is triggered by cooler temperatures and shorter days which are characteristic of autumn.
Among birds, an example of a larder-hoarder is the acorn woodpecker (Melanerpes formicivorus) of western North America. During the summer, acorn woodpeckers collect acorns and store them in the branches of dead trees, drilling a hole for each acorn (Figure 11). Where oak trees are abundant, acorn woodpeckers form very stable social groups, communally defending their larders, which provide group members with food during the winter.
The time for which food is stored varies from one species to another and depends, in part, on its perishability. Coal tits (Parus ater) store insects, having removed the head and the gut, in crevices in tree bark for a few hours. They also store pellets, each one consisting of 20-50 aphids compressed together. Owls store mice for a matter of days and jays and crows store seeds for a year. Ravens store meat for several weeks during the winter, burying it under snow, which keeps it frozen and so delays decay.
The energy that animals invest in food hoarding, and the importance of their cache for their winter survival is illustrated by the following observations:
American biologist Bernd Heinrich observed an individual jay cache 127 food items in a single day.
Individual Clark's nutcrackers (Nucifraga columbina), an American bird, collect up to 95 pine seeds at a time and hide them in locations as much as 22 km from their nest.
Clark's nutcrackers breed in late winter, when there is no food available, and are totally reliant on cached food to rear their young.
Scatter-hoarding presents animals with a problem: if they hide their caches from competitors, how do they find them themselves? Leaving clues, such as scent marks, would lead rivals to the hidden food, and so they must rely on memory. Thick-billed nutcrackers (Nucifraga caryocatactes) store 15-20 hazelnuts in each cache and are able to find them even after thick snow has covered them. Detailed observations of birds in winter suggest that some species are able to memorise the exact location of several thousand stored food items over several months. David Macdonald of Oxford University studied caching in the red fox (Vulpes vulpes) by rearing a pet vixen which he took for walks in the countryside on a long lead. She frequently cached food items that he gave her, burying them in the ground. Over the course of several weeks, she subsequently found 96% of them and only rarely did she dig in places where she had not hidden food.
How do we know that animals rely on what appear to be extraordinary feats of memory to find their food caches? An alternative explanation is that they leave some kind of marker that neither their competitors nor humans can detect. Such an issue can only be resolved by carrying out experiments. Caching behaviour has been studied in American chickadees (Parus spp.) and European marsh tits (P. palustris). Captive-bred birds were trained to feed and hide food in an aviary containing artificial trees that had holes drilled in them, covered with Velcro lids. Opening these lids was very similar to the birds' natural behaviour of stripping bark off trees to find food. In the autumn, the birds spontaneously started to cache seeds in these holes. In a typical experiment, an individual bird was given 15 seeds to hide among a total of 72 open holes. When the bird had left the aviary, the seeds were removed and all the holes were covered up. Twenty four hours later, the bird was allowed back into the aviary. With very few errors, it visited the 15 holes where it had stored seeds the previous day, ignoring the others.
Question 6
Why did the experimenters remove the seeds before the bird was allowed back into the aviary?
Answer
To eliminate any possibility that the bird was using any cues coming directly from the seeds, such as odour.
Scatter-hoarding requires a remarkable memory for the location of a large number of items, a process called spatial memory. Anatomical studies of the brains of birds have revealed that the part of the brain that is involved in spatial memory, called the hippocampus, is relatively larger in species for which scatter-hoarding is particularly important. For example, the hippocampus of the marsh tit (Parus palustris) is significantly larger than that of its close relative, the blue tit (P. caeruleus), which is not a scatter-hoarder.
Changes in social behaviour
For some animals living in temperate habitats, the onset of winter also brings about a marked change in their social behaviour. Most noticeably, many birds, having spent the spring and summer as breeding pairs, being highly aggressive towards all members of the same species, other than their mate, become gregarious and form large, cohesive flocks in which individuals behave cooperatively in a number of ways. As with other biological characters, sociality confers both benefits and costs on individuals.
A major benefit of flocking is that it reduces an individual's risk of being killed by a predator. There are three main reasons for this protection. Firstly, flocks detect predators more effectively than single birds. Secondly, predators become confused when attacking prey gathered closely together. Thirdly, members of flocks may defend themselves effectively against a predator even though, individually, they are not strong enough to do so.
Being in a social group increases feeding efficiency in other ways. Firstly, individuals gain information about the whereabouts of food by observing their fellow flock-members. Secondly, animals in groups can often disturb hidden animal prey more effectively than single individuals. Thirdly, predators in groups can hunt and kill prey that are too large for them to cope with on their own. Studies of captive great tits (Parus major), using an aviary similar to that described earlier, showed that individual tits looking for hidden food found it more quickly when released into the aviary in a small flock. Individuals observed one another and avoided places where other birds had not found food, concentrating their search instead on places similar to those where other birds had located food.
For some animals, joining a social group in winter can reduce heat loss, especially on cold nights. For example, pallid bats (Antrozous pallidus) expend less energy during roosting when they roost huddled together than they do if they roost alone.
There are two major costs to becoming gregarious in winter. First, close association between individuals increases infection rates by parasites and pathogens. For example, in colonies of American prairie dogs (genus Cynomys), there is a positive correlation between the number of animals in a colony and the abundance of external parasites. Secondly, living in a group brings animals closer together, increasing the likelihood that they come into competition over food and other resources. This cost is borne particularly by smaller and weaker individuals, which generally lose in competitive interactions with other group members.
1.3.3 Summary of Section 1.3
Coniferous trees are an example of plants that remain active during winter, with adaptations such as reduced water loss.
Endothermy allows some birds and mammals to remain active during cold winters, but places physiological and energetic demands on the organism, e.g. the need to maintain a high metabolic rate and/or reduce heat loss.
Bird plumage and mammal hair are highly effective insulators, reducing heat loss in winter. Heat loss is also controlled by heat exchange mechanisms and reduction in blood flow near the body surface.
The energy needed to sustain a high metabolic rate may be stored through either physiological and biochemical processes (adipose tissue) or changes in behaviour (hoarding or caching of food).
Some animals change their social behaviour during winter, becoming more gregarious.
1.4 Strategy 2: dormancy in winter ('opt out')
1.4.1 Deciduous trees
During the winter months, a combination of factors, including lower temperatures, reduced light intensity and shorter days, means that plants can only photosynthesise at a slow rate and for restricted periods. As a result, photosynthesis cannot produce energy as fast as respiration expends it. In addition, water is often in short supply because of freezing, and so plants that do not have adaptations to conserve water, as conifers do, would lose water. Deciduous trees avoid these problems in winter by dropping all their leaves and shutting off photosynthesis. Before they do so, they dismantle the photosynthetic apparatus in their leaves and withdraw many of the constituents to their branches, trunks and roots.
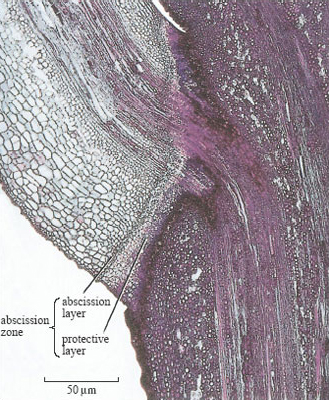
Thus, in the autumn, sugars, amino acids and such minerals as nitrogen, phosphorus and potassium are transported from the leaves to woody tissues. Chlorophyll is broken down and the products are also withdrawn from the leaves. It is this process that causes leaves to change colour in the autumn. The breakdown of chlorophyll leaves behind other pigments, such as orange carotenes and yellow xanthophylls, which are normally hidden by the green chlorophyll. Once as many nutrients as possible have been withdrawn from a leaf, an abscission zone forms where the leaf stalk (petiole) meets the stem (Figure 12). Here the vessels that supply water and nutrients to the leaf are closed off and the leaf detaches, leaving a protective covering of cork over the scar. Leaf abscission is controlled by a complex system of hormones, responding to lower temperatures and light intensity and to shorter day length.
1.4.2 Winter storage in plants
Many plants that survive winter in a dormant state form storage organs below the ground which store nutrients during the winter, the rest of the plant withering away. Storage organs come in a variety of forms, including tap roots, bulbs, corms, rhizomes, root tubers and stem tubers (Figure 13). In the carrot, the root is greatly enlarged into a fleshy tap root; the bulbs of onions are modified leaves; crocus corms, iris rhizomes and dahlia tubers are modified stems, and the tubers of potatoes are modified tips of underground stems.
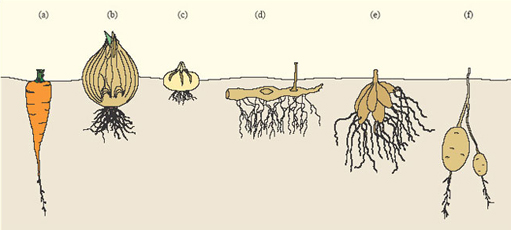
Unlike animals, few plants store energy reserves as fats (lipids). Those that do, generally store fats in seeds or fruits, a good example of the latter being the avocado (Persea americana). Storage in root organs is generally in the form of starch. Because they bind water, carbohydrates prevent desiccation of the storage organ and also reduce its freezing point, acting as 'antifreeze'. Plant storage organs provide ready-made larders for those herbivores that remain active in winter.
The root of a carrot serves as a storage organ, enabling the plant to complete its two-year life cycle. The storage organs of many plants are, however, also a means of asexual or vegetative reproduction. For example, the rhizomes of irises grow and branch and, as older parts of a rhizome die, two or more new plants are formed from the parts that are left. The tubers of a potato plant can each grow into a new plant and the bulbs of such plants as onions and daffodils (Narcissus sp.) divide to produce new bulbs and thus new plants.
1.4.3 Freeze tolerance in ectothermic vertebrates
In Britain, the vertebrate class Amphibia is represented by frogs, toads and newts. Amphibians are ectotherms, meaning that they are unable to generate large quantities of heat within their bodies, so their body temperature is close to that of their surroundings. The majority of amphibian species avoid the lethal consequences of being frozen, by digging their way under a large object, such as a rock, or deep into the soil, below the level that is penetrated by frost. There are some species, however, that have evolved a physiological response to very cold weather that enables them to survive the winter on or close to the ground surface. Examples include the American wood frog (Rana sylvatica) and the Asian salamander (Hynobius kyserlingi), both of which have distributions that extend far north of the Arctic circle. What they do is to infuse their tissues with antifreeze.
In the wood frog, the onset of cold causes the animal to become immobile. As the temperature falls below 0°C, water in its toes begins to freeze. Within 10-15 minutes of freezing, glycogen stored in the liver is converted into soluble glucose which is released into the bloodstream, whence it finds its way into the cells and the extracellular spaces (Figure 14). The dissolved glucose lowers the freezing point of water, as antifreeze does in a car's radiator, preventing the formation of ice crystals and any consequent movement of water out of living cells.
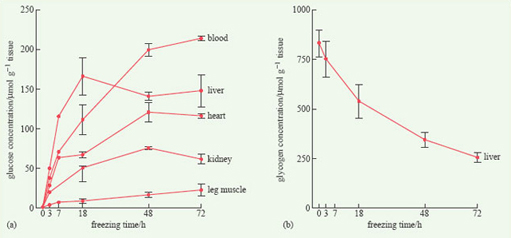
Whereas the wood frog uses glucose as an antifreeze, the Asian salamander and the grey treefrog (Hyla versicolor) use glycerol, suggesting that this adaptation may have evolved independently in a number of amphibian species. Freeze tolerance allows these amphibians to survive freezing conditions for one or two weeks. It is not their only adaptation for surviving the winter; in the wood frog, for example, breathing ceases and the heart stops beating at very low temperatures.
Some ectothermic vertebrates rely on supercooling to survive short periods of cold temperature (see Section 1.2.1). For example, the spring lizard (Sceloporus jarrovi), living in the Arizona desert, survives very cold nights by supercooling. This strategy is risky, however, and many lizards die as a result of becoming frozen. Allowing its tissues to supercool is not a viable option for a frog or salamander; living in damp places, they are virtually certain to be in contact with ice crystals which act as nucleation points.
As well as enabling them to survive frosty conditions, the capacity to tolerate extreme cold confers other advantages on some amphibians. Many breed in temporary ponds that dry up early in the spring or summer, making it advantageous for breeding adults to migrate to ponds as early as possible in the spring. Early breeding maximises the time available for the aquatic egg and larval stages to be completed before a pond dries up. Some species, such as the American blue-spotted salamander (Ambystoma laterale) migrate to breeding ponds while snow is still on the ground, giving them an advantage over other salamander species that do not start to breed until the weather is warm.
1.4.4 Hibernation in mammals
Many animals become inactive for periods of varying duration during the winter and there is a diversity of terms used to describe this state, including: sleep, torpor, dormancy, lethargy and hibernation. The word hibernation is often used loosely to refer to general inactivity but, in biology, it refers to a specific phenomenon, sometimes called 'true hibernation'. Hibernation is defined as the condition of passing the winter in a resting state of deep sleep, during which metabolic rate and body temperature drop considerably. It occurs only in certain mammals and one bird species, the poorwill (Phalaenoptilus nuttallii), a North American relative of the nightjar.
The phenomenon of hibernation is one reason why the term homeothermy is going out of fashion, to be replaced by endothermy, because maintaining a stable body temperature is the very opposite of what hibernators do. Instead, body temperature falls, from around 38°C, to about 1°C above ambient temperature, which is often close to 0°C. At the same time, a hibernator's metabolic rate falls to as little as 1% of its normal value. The heartbeat becomes slow and irregular and breathing rate also slows.
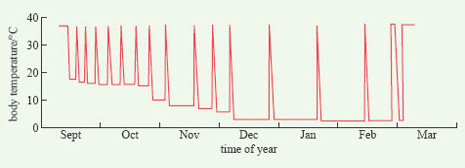
Hibernation is an active process, that is, it is a state which animals enter into, not in response to immediate external conditions, but to internal stimuli. Some species are remarkably precise and predictable. For example, the arctic ground squirrel (Spermophilus undulatus) enters hibernation between 5 and 12 October and emerges between 20 and 22 April, regardless of the weather on those dates. This behaviour is in contrast to other winter states such as torpor or lethargy which are immediate responses to current conditions. Brown and black bears, for example, are lethargic during very cold periods but are otherwise active in the winter. A feature of hibernation that distinguishes it from other kinds of winter inactivity is that hibernators can arouse themselves spontaneously and are not dependent on external conditions, such as warm temperatures, to do so. The arctic ground squirrel is described as an obligate hibernator because it hibernates every winter. There are some mammals that are categorised as facultative hibernators, entering hibernation in response to very cold weather and poor food supply. The North American pocket mouse (Perognathus californicus) is a facultative hibernator.
True hibernation only occurs in relatively small mammals, though not all small mammals living in temperate habitats hibernate in winter, as we have seen. The largest mammal to hibernate is the marmot, which weighs about 5 kg. There are several reasons why larger mammals do not hibernate. Firstly, they would warm up too slowly and therefore use too much energy. Secondly, they have a smaller surface area to volume ratio and so can conserve body heat better than smaller species. Finally, they are better able to carry a thick coat (Section 1.3.2) and sufficient adipose tissue to last through the winter. Hibernators are mainly found in the orders Rodentia, Chiroptera (bats) and Insectivora. The hedgehog (Erinaceus europaeus) is an example of a hibernating insectivore; in Britain, it hibernates from October/November to March/April. Note that although hedgehogs are in the order Insectivora they do not just eat insects!
The physiological features that are characteristic of hibernation are not maintained throughout the winter. Rather, the animal wakes up at intervals, its temperature and metabolic rate increasing to near-normal levels (Figure 15). The function of this periodic arousal is not wholly clear. Some species, such as the chipmunks (genus Tamias) eat from stored food reserves during arousal periods, but many others do not. Most species urinate and defecate, move about and change their position, suggesting that arousal provides an opportunity for various essential physiological processes to be performed and to prevent the animal becoming moribund. From detailed measurements of Richardson's ground squirrel (Spermophilus richardsonii) in the laboratory, it has been calculated that, during the relatively brief periods of arousal (Figure 15), an individual expends 83% of all the energy that it uses up during the entire hibernation period.
Hibernation requires internal energy reserves in the form of adipose tissue and hibernators typically feed intensively prior to winter, building up their fat stores. Some species, such as the edible dormouse (Glis glis), switch to a carbohydrate- and lipid-rich diet, e.g. seeds, at this time. A characteristic of hibernating mammals is that they possess larger quantities of a particular kind of adipose tissue called brown adipose tissue (BAT). This tissue gets its name from its dark colour, which is due to the larger numbers of blood capillaries that permeates it and the high concentration of mitochondria within the cells. BAT is rich in mitochondria with special properties that enable it to oxidise fatty acids and/or glucose to produce heat very rapidly. BAT deposits are found around some internal organs and between the shoulder-blades of hibernators and their function is to generate body heat very rapidly, especially during periods of arousal.
Hibernation might seem to be a safe, and rather agreeable way to spend the winter but, for some species, it is fraught with danger. For Belding's ground squirrels (Spermophilus beldingi) living at high altitude in Tioga Pass, California, hibernation lasts 7-8 months. Two-thirds of all juveniles, hibernating for the first time, and one-third of adult animals die during hibernation. Some die because their fat reserves run out before the end of hibernation; others are dug up and eaten by predators.
Some mammals spend the winter in groups, huddled together during periods of dormancy, and so conserve body heat. North American raccoons (Procyon lotor), for example, spend dormant periods in communal dens. Many species of bats hibernate communally. During hibernation, the body temperature of some bats can fall below 0°C. In the autumn, they build up fat reserves that represent as much as a third of their total mass. During the winter, bats arouse themselves from hibernation to excrete and sometimes also to move to a new roost. A critical factor for hibernating bats is that roosting sites have high humidity and some populations have to migrate quite large distances to find suitable places, such as caves and hollow trees.
For some mammals, hibernation is closely associated with other important activities, notably reproduction and dispersal. Consequently, energy reserves may have to support more than one activity. For example, brown bears living at northern latitudes mate in the autumn and give birth to their cubs during winter lethargy. Edible dormice and some bats mate immediately after the end of hibernation. (In some species of bats, males wake up first and mate with the females before they have woken up!) The link between what animals do in winter and their reproductive cycles was discussed in Section 1.2.3.
Natal dispersal is the permanent departure of an individual from its place of birth, usually at the end of the breeding season. It is an important part of the life history of many animals, especially mammals, and tends to be sexually dimorphic, males dispersing further than females. Natal dispersal is potentially both hazardous and energetically expensive; dispersing animals tend to be vulnerable to predators and, being on the move, have little time to feed. Dispersal therefore requires internal energy reserves in the form of fat, the very same reserves that they later need to survive the winter. There may thus be a trade-off in the allocation of energy reserves to dispersal and to hibernation.
Scott Nunes of Michigan State University has studied dispersal and hibernation in Belding's ground squirrels in a locality where hibernation lasts for eight to nine months of the year. Young males typically disperse after the summer breeding season but show much variation in the extent to which they do so, with fatter males being more likely to move out of the natal area. In years when breeding is delayed, dispersal is inhibited; instead, young males remain near the natal area, building up their fat reserves prior to hibernation. The findings of this study are summarised in Figure 16.
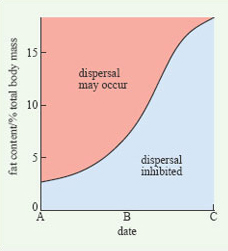
If young males are ready to disperse early (date A in Figure 16), they leave with relatively small fat reserves. In this situation, they have time to travel further to a new area and then build up fat reserves before hibernation. If dispersal is delayed (dates B and C), males do not disperse unless they have built up a threshold level of fat reserves; the value of this threshold increases as winter approaches. In other words, the trade-off between dispersal and hibernation is resolved by hibernation suppressing dispersal, unless an animal exceeds a certain fat level. After date C, dispersal is inhibited regardless of the size of the fat reserves.
1.4.5 Summary of Section 1.4
Deciduous trees avoid the problems of winter by shedding their leaves.
Plants can store nutrients over winter in a variety of structures.
Amphibians have evolved behavioural responses (e.g. burying themselves) and physiological responses (e.g. different types of antifreeze in the body fluids) to winter.
Hibernation occurs only in certain small mammal species and one species of bird and is accompanied by marked physiological and behavioural changes.
Prior to hibernation, animals build up their fat reserves and frequently possess larger amounts of brown adipose tissue than non-hibernators.
There may be a trade-off between hibernation and dispersal in some animals.
1.5 Strategies 3 and 4: juvenile survival and migration
1.5.1 Juvenile survival
For organisms that are able to complete their life cycles within a year there is the possibility of spending the winter in various juvenile stages. We have already considered annual plants, the adults of which may die before the onset of winter, with seed not germinating until the spring. Surviving the winter as seeds has the advantages that the seeds are robust, and because they have a low water content they are less affected by freezing temperatures. Disadvantages of this strategy include the possibility that the seeds may be consumed by ground-feeding herbivores and that newly germinating plants may lose out in competition with annuals that germinated in the previous autumn.
Butterflies are good examples of insects that survive the winter in a variety of immature stages. Indeed, in Britain, different butterfly species overwinter at each of the juvenile stages of the life cycle, i.e. as eggs, larvae and pupae, or as adults.
Table 1.2 lists the numbers of species, categorised by butterfly family, that overwinter in each of these four stages in Britain.
| Juvenile stage | Adult | |||
|---|---|---|---|---|
| Butterfly family | Egg | Larva | Pupa | |
| Satyridae | 0 | 11 | 0 | 0 |
| Nymphalidae | 1 | 9 | 0 | 4 |
| Lycaenidae | 6 | 7 | 2 | 0 |
| Pieridae | 0 | 0 | 5 | 1 |
| Hesperiidae | 2 | 5 | 1 | 0 |
| other | 0 | 0 | 2 | 0 |
| total | 9 | 32 | 10 | 5 |
Question 7
Based on Table 1.2, what is the predominant overwintering stage of butterflies?
Answer
Larvae (caterpillars), occurring in 32 out of 56 species (57%).
There appear to be some phylogenetic patterns in overwintering strategy amongst the butterflies, i.e. some butterfly families in Britain are restricted to certain strategies.
Question 8
Which butterfly families are restricted to overwintering at one immature stage in Britain?
Answer
The Satyridae (browns) only overwinter as larvae and the Pieridae (whites) overwinter as pupae.
In other families, such as the Lycaenidae (blues) and Hesperiidae (skippers), there is a mixture of overwintering strategies. However, these butterfly families are large and heterogeneous, and more careful inspection of subfamilies indicates phylogenetic patterns. For example, most hairstreaks (family Lycaenidae) overwinter as eggs. Across families, it seems there are common factors in the types of species that overwinter as a particular stage. For example, all of the species that overwinter as eggs have only one generation a year and are on the wing (and therefore breeding) later in the year, mainly July and August.
1.5.2 Strategy 4: migration ('go away')
About 40% of the bird species that breed in Britain do not spend the winter there but migrate south, some to southern Europe, others much further afield. The swallow (Hirundo rustica), for example, may migrate as far as the Cape of southern Africa. From one perspective, migrants are European species that avoid the northern winter by migrating to a less severe environment. On the other hand, the swallow can also be regarded as an African bird that migrates to northern latitudes to breed. Why should an African bird migrate thousands of miles to breed, exposing itself to obvious risks? Swallows suffer 67% annual mortality, much of it occurring during migration. Long-distance migration also involves a huge energetic cost. The answer is that northern latitudes provide very good breeding habitats for birds. In late spring and summer these habitats support a rich food supply, in the form of seeds, fruits and insects, and there are relatively fewer herbivorous and insectivorous species living at northern latitudes, so that competition for food with which to rear their young is relatively low. Evidence for this conclusion comes from data on the clutch size of birds of the same or related species breeding at different latitudes. Temperate-breeding birds lay much larger clutches than similar species breeding at more southerly latitudes. This effect is also seen within species; for example, in the European robin (Erithacus rubecula), mean clutch size is 6.3 eggs in Scandinavia, 5.9 in central France, 4.9 in Spain, 4.2 in north Africa and 3.5 in the Canary Islands.
Birds are not the only animals that migrate. Among mammals, some populations of caribou or reindeer (Rangifer tarandus) make mass migrations of more than 1000 km, covering up to 150 km per day, in search of good grazing. Some insects also migrate over long distances. For example the monarch butterfly (Danaus plexippus) migrates from Canada and northern USA to Mexico, flying 120 km per day. Some 30% of the monarch population, however, does not migrate but hibernates during the winter.
Migration in birds requires considerable physiological preparation. Some time before they leave, migrants increase their feeding rate and lay down fat reserves, amounting to between 20 and 50% of total body mass, depending on the species. At the top of this range are the ruby-throated hummingbird (Archilochus colubris), which flies across the Gulf of Mexico, and the sedge warbler (Acrocephalus schoenobaenus), which migrates from Britain to west Africa. Both these species complete their migration in a single sustained flight. Species that carry smaller loads of fat typically fly in a series of stages, stopping to feed and put on weight along the route. The rate at which migratory birds put on weight prior to migration is remarkable. Whitethroats (Sylvia communis) preparing to migrate from Sweden increase their food intake by about 70%, their body mass increases by 7% per day, and they depart 50% heavier than normal.
Prior to migration, smaller birds build up larger fat reserves, relative to their body size, than do larger birds. This size effect is related to a measure of the amount of power that a bird can generate, called the power margin, which is defined as the difference between the maximum power than can be developed by the flight muscles and the power required for unladen (with no extra fat) level flight at a standard speed in still air. A large bird such as a swan has a small power margin, so swans need to build up speed before they can take off, and, once airborne, they climb rather slowly. Small birds, with a large power margin, can take off vertically and climb very rapidly (Hedenstrom and Alerstam, 1992). The small power margin of large migrants, such as white storks (Ciconia ciconia) which migrate from Europe to Africa and back, means that they cannot carry large amounts of fat to support long flights. They economise on fuel, to some extent, by soaring on thermals, rather than using flapping flight, but these habits constrain them to routes that do not cross the sea. Storks migrate either over Gibraltar in the west, or over Israel in the east, depending on where they breed. They also make frequent stops to feed.
As birds put on weight prior to migration, they start to show migratory restlessness. Birds held in captivity just before the migration season do a lot of hopping about and experiments have shown that this hopping is not random, but is oriented in different directions in spring and autumn (Figure 17).
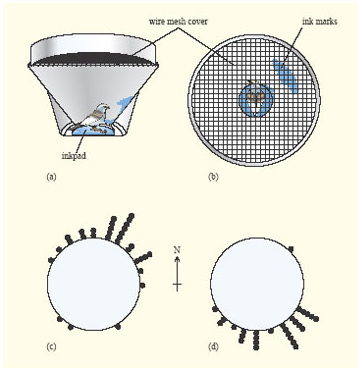
Experimental studies of captive birds have revealed that the proximate factors that stimulate increased feeding prior to migration and migratory restlessness are decreases in the L : D ratio (i.e. longer nights) and, in some species, a circannual clock.
To complete their life cycle, migratory birds make at least two journeys: outwards to the wintering site and a return journey next spring back to the breeding site. A major determinant of reproductive success for many temperate bird species is the date on which they start to breed. In the majority of species, pairs that start to breed early in the spring fledge more young. There are several reasons for this correlation, the relative importance of which varies from species to species. Firstly, early breeders generally secure better territories, in terms of the abundance of food that they contain. Secondly, an early start often means that a pair has time to produce and rear a second clutch in the same season. Thirdly, in some species the earlier breeders are better synchronised with their environment, such that the time of peak food requirement (i.e. during feeding of the young) is synchronised with the time of maximum food availability.
A recent study of the American redstart (Setophaga ruticilla), suggests that a further factor could be important. It was found that the earliest birds to arrive at breeding sites were in better condition than later birds, some of which arrived up to a month later. This study investigated the tropical wintering habitat of this species and found that the early-arriving, good-condition birds had wintered in better habitats than later arrivers. This study therefore suggests that the quality of winter habitats may be an important determinant of fitness for migratory birds. It also reinforces the point that, in considering the success of organisms, it is important to consider all parts of their life cycle.
1.5.3 Summary of Section 1.5
Some annual plants and insects can spend the winter at juvenile stages, such as seed, egg, larva or pupa. Butterflies in Britain display a variety of juvenile overwintering strategies.
Migration often results in high mortality, but completion of the journey results in higher breeding success, due to increased availability of food and fewer competitors.
Birds increase their body mass, sometimes by up to 50%, prior to migration. The body mass increase is related to the power margin of the birds.
Early arrival of migratory birds at the breeding site confers important advantages on those individuals.
1.6 Conclusion
This course has presented an overview of the ways in which organisms living in temperate habitats are adapted to survive the winter. The course has shown how a limited set of environmental changes associated with the onset of winter can lead to a diversity of adaptations and therefore a large diversity of species.
On the basis of the examples discussed in this course, we can identify four factors that contribute to the diversity of adaptive strategies for coping with winter.
Alternative strategies and trade-offs For any one environmental 'problem', there may be two or more solutions that may be appropriate in certain species under certain conditions. Thus, remaining active, becoming dormant and migrating are all viable options for long-lived organisms to cope with winter. Among animals that become dormant, building up reserves in the form of a larder is an alternative to depositing lipids in adipose tissue. These adaptive solutions may be thought of as being traded off, i.e. natural selection may lead to an organism adopting one particular adaptive solution. This effect is reinforced by an organism's evolutionary history (see below).
Phylogeny The evolutionary history of different groups of organisms has equipped them with a range of adaptations that predispose them towards one strategy rather than another. For example, endothermy and thick fur make hibernation a viable strategy for mammals, wings make long-distance migration a viable strategy for birds.
Size Body size can have a marked effect on what organisms do in winter. Dying back and overwintering as an underground storage organ is a viable option for small plants, but not for large woody ones; hibernation is possible for small to medium-sized mammals but not for large ones.
Life history The particular life history of organisms allows them to survive the winter in different ways. Thus annual plants have the option of overwintering as small plants or seed whilst herbaceous perennial plants have the extra option of surviving the winter underground. As life history is itself subject to natural selection, it is another example of evolutionary history restricting the options of adaptive solutions.
A fifth factor, which we have not had the space to explore in this course, is coevolution. Through coevolution, adaptations by one group of organisms create opportunities for other groups. Thus, in the context of this course, if it were not for the fact that many plants store food over the winter, there would be little opportunity for many herbivores to remain active in winter. Coevolution implies that, if selection favours herbivores that can exploit underground storage organs, then, equally, selection also favours plants that are able to defend these resources.
2 SAQs
Question 1.1
Read the following account and then state which strategy from Table 1.1 it best fits.
While freezing is lethal for most organisms, one group of organisms is unaffected by it. Water-bears or tardigrades (phylum Tardigrada) are tiny creatures, measuring only 0.05-1.5 mm in length (Figure 1). They live in very small water bodies that are liable to dry up in summer and winter. In dry conditions, tardigrades lose most of their water, shrivel up and transform into barrel-shaped 'tuns', in which state they can survive for ten years or more. In experiments, tardigrade tuns have survived being chilled to −272°C for eight hours and being heated up to 150°C.
Answer
This response agrees with the 'opt out' strategy (2) in which organisms maintain an inactive existence for the duration of the hostile period.
Question 1.2
A crucial question that arises in relation to photoperiodism is whether it is the duration of the light period, the dark period, or both to which organisms respond. Consider the following experiments. Many plants and animals have been kept under artificial L : D regimes in which one or other part of the cycle is briefly interrupted. For example, the dark period can be interrupted by turning on the lights for a short period. Typically, short light periods during the dark period, in some instances as short as one minute, can completely change the flowering of plants or the behaviour of animals, but comparable short dark periods during the light period have no effect.
Do these experiments demonstrate that organisms are sensitive to the duration of the light period, or the dark period or both?
Answer
This experiment provides clear evidence that organisms respond to the duration of the dark period, not to the duration of the light period.
Question 1.3
Why should the fact that coniferous needles are shaped like cylinders be advantageous in reducing water loss?
Answer
A needle, which is a long, thin cylinder, has a smaller surface area relative to its volume than a flat blade, so its rate of water loss per unit mass is correspondingly lower.
Question 1.4
The kestrel (Falco tinnunculus) is a solitary hunter all year round. In Britain, it feeds primarily on the common vole (Microtus arvalis), which is also active in winter. Because their prey are relatively large and very nutritious, kestrels do not need many meals each day and the shortness of winter days does not limit their hunting success. One change of behaviour that kestrels do show in winter is to switch from flight-hunting, in which they look for prey while hovering above the ground, to perch-hunting. The latter is much less energetically expensive and involves sitting patiently on a branch until an active vole is spotted on the ground. Why should perch-hunting be favoured in winter?
Answer
Perch-hunting is favoured in winter because vegetation is lower and many trees are leafless, so small mammals are more easily seen from a perch.
Question 1.5
Which of the four responses to winter is observed in each of the following: bats, edible dormouse, caribou, deciduous trees, annual plants, swallows and conifers?
Answer
Conifers remain active during winter (strategy 1). Bats, dormice and deciduous trees become inactive during winter (strategy 2). Most annual plants survive the winter only as seeds (strategy 3, although some species germinate in autumn and overwinter as juvenile plants). Swallows and caribou migrate (strategy 4).
Conclusion
This free course provided an introduction to studying Environment & Development. It took you through a series of exercises designed to develop your approach to study and learning at a distance, and helped to improve your confidence as an independent learner.
Take the next step

If you enjoyed this course, why not explore the subject further with our paid-for short course, Science: the weather?
References
Acknowledgements
The content acknowledged below is Proprietary (see terms and conditions) and is used under licence.
Course image: Vladimir Yaitskiy in Flickr made available under Creative Commons Attribution-NonCommercial-ShareAlike 2.0 Licence.
Grateful acknowledgement is made to the following sources for permission to reproduce material in this course:
Figure 3 Bronson, F.H. (1987) 'Environmental regulation of reproduction in rodents', in Crews, D. (ed.) Psychobiology of Reproductive Behavior, p. 209, Prentice-Hall Inc., a Pearson Education Company;
Figure 6, 9 Adapted fro Irving, L. (1996) 'Adaptations to cold', Scientific American, 214 (1), p.96, by permission of the Executrix of the Estate of Bunji Tagawa;
Figure 7 Adapted from A Dictionary of Birds (1985) p. 207, T. & A. D. Poyser Limited, by permission of Academic Press (London) Limited, © The British Ornithologists' Union, 1985;
Figure 8 Pough, F. H., Heiser, J. B. and McFarland, W. N. (1996) Vertebrate Life, 4th edn, © 1996 by Prentice-Hall, Inc. Simon and Schuster/A Viacom Company, Upper Saddle River, NJ;
Figure 11 Halliday, T. (1980) Sexual Strategy, Oxford University Press;
Figure 12 From Biology of Plants by P. Raven, R. F. Evert and S. E. Eichhorn, © 1999 by W. H. Freeman and Company/Worth Publishers. Used with permission;
Figure 14 Pinder, A. W., Storey, K. B. and Ultsch, G. R. (1992) 'Estivation and hibernation', in Feder, M. E. and Burggren, W. W. (eds) Environmental Physiology of Amphibians, The University of Chicago Press, © 1992 by The University of Chicago. All rights reserved;
Figure 15 From L. C. H. Wang (1978) Strategies in Cold: Natural Torpidity and Thermogenesis, L. C. H. Wang and J. W. Hudson (eds), Academic, New York;
Figure 16 Nunes, S. et al. (1998) 'Body fat and time of year interact to mediate dispersal behaviour in ground squirrels', Animal Behaviour, 55 (3), p. 612, Academic Press (London) Limited;
Figure 17a and b Illustration by Adolph E. Brotman in Emlen, S. T. (1975) 'The stellar-orientation system of a migratory bird', Scientific American, August 1975;
Figure 17c and d Emlen, S. T. (1975) 'The stellar-orientation system of a migratory bird', Scientific American, August 1975
Adapted fro Irving, L. (1996) 'Adaptations to cold', Scientific American, 214 (1), p.96, by permission of the Executrix of the Estate of Bunji Tagawa
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
Copyright © 2016 The Open University