Birth of a drug
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Tuesday, 23 April 2024, 3:25 PM
Birth of a drug
Introduction
This course examines how organic chemistry is used within the pharmaceutical industry to develop new drugs. You will investigate the process of drug development by following one new product through the intial process and research programme.
This OpenLearn course provides a sample of Level 3 study in Science.
Learning outcomes
After studying this course, you should be able to:
explain the criteria that must be satisfied before starting new drug development
say how an understanding of the pharmaceutical background is a necessary basis for the design of the new drug
understand the strategies used in the research programme and how they led eventually to the development of a new drug.
1 The chemical industry
The chemical industry is one of the major contributors to the economies of advanced industrial nations.
The importance of organic chemistry in the pharmaceutical industry becomes apparent in the light of the fact that in the UK alone some £2.2b was spent in 1997 on research and development. Literally thousands of different compounds have to be synthesised and tested in the search for the one that will prove to be a successful and valuable therapeutic agent. As a result, the average cost of developing a new drug is now around £350m. And because of the increasingly stringent safety requirements of the regulatory authorities, the development time has risen from an average of three years in 1960 to 12–15 years today. Given that the patent life of any new drug is around 20 years, this leaves a relatively short time for the pharmaceutical company to recoup its investment. So the stakes are high; but equally, the rewards are great. The worldwide sales of so-called ‘ethical pharmaceutical products’ in 1998 exceeded £150b. Table 1 shows the ten most prescribed drugs in the USA, with all of them exceeding annual sales of £1b. In contrast, the top-selling drug in 1989, Glaxo's Zantac, only managed worldwide sales of £900m!
| Brand Name | Generic Name | Company | Treatment/Use | Sales/£m yr−1 |
|---|---|---|---|---|
| Losec | omeprazole | AstraZeneca | ulcers | 2490 |
| Zocor | simvastatin | Merck | cholesterol lowering | 2470 |
| Prozac | fluoxetine | Eli Lilley | depression | 1750 |
| Norvasc | amlodipine | Pfizer | hypertension | 1600 |
| Vasotec | enalapril | Merck | hypertension | 1500 |
| Claritin | loratadine | Schering | antihistanmine | 1440 |
| Lipitor | atorvastatin | Warner–Lambert | cholesterol lowering | 1375 |
| Zoloft | sertraline | Pfizer | depression | 1150 |
| Paxil | paroxetine | SmithKline Beecham | depression | 1100 |
| Augmentin | amoxicillin/potassium clavulanate | SmithKline Beecham | antibiotic | 1000 |
2 Video activity
The video clip included in this section looks at the development of one particular drug. It examines the complex multidisciplinary process that was involved in its discovery. Before you watch the video, read through the Pre-viewing notes.
Now watch the video clips and make notes on the process involved in the development of the drug.
Now you have watched the clips, try answering the following questions. The answers are provided within the content of Section 5.
What is the role of noradrenaline in controlling blood pressure?
Is noradrenaline an agonist or an antagonist for alpha receptors?
What is the difference between an alpha1 receptor and an alpha2 receptor?
In what ways did prazosin differ in its pharmacological effects when compared with the early alpha blockers?
Why was research in this area discontinued?
What development caused the research programme to be resumed?
What was the major goal in designing an improved drug?
What was the strategy adopted in the ensuing research programme?
What part of the prazosin and doxazosin molecules seemed to be essential for alpha1 selectivity; what part seemed to be associated with increased activity and effectiveness at reducing blood pressure; and what part seemed to be linked to duration of action?
3 Reading activity
You will shortly be asked to read through a research paper published in the Journal of Medicinal Chemistry, in which the synthesis and structure–activity relationships of doxazosin and related compounds are described. It has been provided:
(a) to show you how the results of such a research programme are reported to the wider scientific community. Note that once the essential steps in the synthesis of a new drug have been patented, pharmaceutical companies (and indeed companies in the fine-chemical industry generally) encourage their research workers to publish the results of their work just as researchers in academic institutions do;
(b) to provide a more detailed explanation of the chemical background to the development of doxazosin;
(c) to show in particular how the syntheses of new, hitherto unknown, compounds are reported, with details of the route(s) and experimental conditions employed, and sufficient data reported to establish unambiguously the structures of the new compounds.
In reading this paper you should not attempt to commit to memory the details of the syntheses described, nor should you let yourself become bogged down by some of the pharmacological terms. Instead, concentrate mainly on the chemistry. Note that norepinephrine is just the alternative name for noradrenaline used in the USA.
Click to open a Research paper from the Journal of Medicinal Chemistry
4 Summary of video
At the beginning of the video clip, Dr Simon Campbell pointed out that, in the development of any drug, there are a number of criteria that must be satisfied. There must be a clinical need – a medical condition or disease that requires effective treatment. Secondly, there has to be a commercial opportunity; because drug development is so expensive, pharmaceutical companies are less likely to invest resources in developing new therapeutic agents where there is already an adequate range of suitable drugs available. Thirdly, at the stage of planning the research project, it is essential that there is some pharmacological understanding of the underlying cause of the disease that the drug will be used to treat. Fourthly, there has to be a chemical starting point, often a compound already known to have the desired pharmacological activity to some degree. It could be that, if such a compound is not available, it may be necessary to generate new chemical starting points de novo, possibly from a compound in the literature. Increasingly, computer-based molecular graphics systems are being used in this process to provide suitable leads, by consideration of the shape and electronic properties of the receptor or enzyme involved, and so designing compounds from first principles.
The development of doxazosin, as described in the video, can be divided into a number of stages: the medical background; the pharmacological background; the project initiation; and the medicinal chemistry strategy.
5 Birth of a drug
5.1 The medical background
Blood pressure is normally tightly controlled in the face of large variations in blood flow required by activities such as vigorous exercise. The diameter of the blood vessels is under the influence of the so-called sympathetic nervous system. Impulses from the brain stimulate the release of a chemical substance, noradrenaline (1), from the nerve-endings close to the vessel walls. The noradrenaline then diffuses to the blood vessel wall, where it interacts with a specific molecular site called an alpha receptor. This interaction results in contraction of the smooth muscle in the vessel wall, and hence constriction of the blood vessel itself. Substances like noradrenaline that bring about biochemical and pharmacological changes are called agonists.
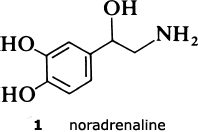
In hypertension, however, control is abnormal and blood pressure is raised. Although the primary cause is unknown, it is thought that the blood vessels become over-constricted, possibly through overactivity of the sympathetic nervous system, with release of greater than normal amounts of noradrenaline, thereby causing an increase in pressure for any given rate of flow.
About 10–20 per cent of the adult population in the UK suffers from hypertension, a condition that is a major contributor to heart disease. So the first criterion is met: there is a medical condition that needs to be treated. The very significant proportion of the population with hypertension also indicates a commercial opportunity for drugs which provide improvements over existing therapy, so fulfilling the second criterion.
5.2 The pharmaceutical background
The existence of alpha receptors had been known for many years, and the obvious approach of trying to block these receptors, and hence prevent the noradrenaline from binding, had already been tried by many research groups. A number of compounds with alpha-blocking activity had been identified, including one called prazosin (2) discovered by Pfizer at their US research laboratories in Groton. Substances such as prazosin which block agonist action are called antagonists.
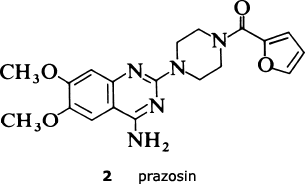
However, none of the early alpha-blockers, with the single exception of prazosin, reduced blood pressure other than transiently; furthermore, the old alpha-blockers usually displayed undesirable side-effects. Prazosin, on the other hand, was shown to be effective at lowering blood pressure and safe in toxicological evaluation, and so in due course was made available for the treatment of hypertension.
Prazosin needed to be taken two or three times a day, whereas, as indicated earlier, a once-daily therapy would be preferable for the treatment of a chronic condition such as hypertension; furthermore, the lack of detailed understanding of the mechanism of action of prazosin (in other words, the answer to the question: why was it effective where other alpha-blockers were not?) was a significant limitation to implementing a rational follow-up programme and, after some initial work, research in this area was discontinued.
5.3 Project initiation
In the early 1970s a discovery was made independently by two different researchers, Klaus Starke in Germany and Salomon Langer in Argentina. Their results showed that there were two types of alpha receptor. The first type, which they called alpha1 is the one already described that is found on the blood vessel wall. The second type, which they called alpha2, is located on the nerve-ending itself. Noradrenaline binds to the alpha2 receptors when the alpha1 receptors are saturated, thereby inhibiting further release of noradrenaline from the nerve-endings. Most importantly, the alpha2 receptors probably have a different shape from the alpha1 receptors and may well interact in a different way with the noradrenaline molecule.
Dr Michael Davey realised that this could provide an explanation of the unusual pharmacological properties displayed by prazosin. He and his co-workers were soon able to show that prazosin was a potent, highly selective, alpha1-blocker, and interacted only very weakly, if at all, with the alpha2 receptor. In contrast, many of the old alpha1-blockers could block both types of receptor thus allowing continued release of noradrenaline, an effect which soon overcame the ability of these drugs to reduce blood pressure. Clearly this new insight satisfied the third criterion of an understanding of the pharmacological mechanism and the fourth requirement, a chemical starting point, was provided by prazosin itself. Here then was the necessary scientific basis to allow the design of an improved drug, one that would only need to be taken once a day.
5.4 The medical chemistry strategy
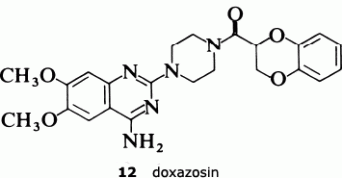
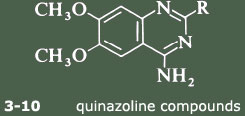
The strategy adopted was to start from first principles. Since prazosin is an alpha1 antagonist, it must compete with the agonist, noradrenaline, for the alpha1 receptor. So the Pfizer scientists decided to compare the structures of the two molecules and look for common structural features that might provide an insight into the way in which they bind to the receptor. One important observation was the fact that, at physiological pH, both compounds would be protonated, noradrenaline at the terminal nitrogen and prazosin at N-1. They then postulated that one active part of the receptor was a polar entity such as a carboxylate group. If this was situated equidistant from the protonated N-1 in prazosin or the nitrogen atom in noradrenaline, so called charge-reinforced hydrogen bonding could take place equally well for both molecules. This model is shown in the reprint as Figure 5. This hypothesis indicated that the substituted quinazoline subunit (Figure 1 below) was an essential structural component in the prazosin series for specific binding to the alpha1 receptor.
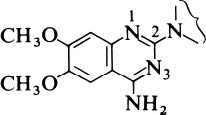
The next step was to investigate the effect of changing the structure of the rest of the molecule. The Pfizer scientists took a series of compounds, each containing the substituted quinazoline sub-unit, but with a variety of substituents attached at the 2-position, and tested them for alpha1 receptor affinity. This did not involve very much synthesis, since Pfizer maintains a chemical repository where every compound made in their laboratories is kept. These samples are catalogued before storing them in what is effectively a compound library for future reference. To a pharmaceutical company this is as valuable a resource as the more conventional document library. Details of each and every compound, including the structure, are then entered into a computer file. This ensures that, when required, retrieval both of the information and of the compound itself is very straightforward. So initial testing of the hypothesis simply involved searching the compound library for compounds containing the required diamino-quinazoline group and screening them for alpha1-blocking activity.
Several compounds containing the crucial substituted quinazoline group were tested both for alpha1 and for alpha2 activity. The 2-substituents covered a wide range, from the simple amino group through complex hetero-cycles.
The tests vindicated the hypothesis completely: none of the compounds showed any alpha2 activity but all had some degree of alpha1 affinity. The results are shown in Table 2. The figures in the first column are related to the concentrations of the compounds necessary to displace a radioactive ligand from the alpha1 receptor, so the smaller the number the more active the compound. All the compounds illustrated, except for 3 and 4, are highly active. But in vitro measurements of alpha1 affinity are only a guide to the effectiveness of the compounds as anti-hypertensive agents in vivo. The stars in the second column indicate how effective these compounds are in lowering blood pressure in rats after oral administration. These results show that the original compound, prazosin (10), still seems to be one of the most effective anti-hypertensive agents. So how to proceed?
The results have confirmed the need for the substituted quinazoline subunit. The presence of a large substituent attached at the 2-position seems to increase activity and to improve the effectiveness in reducing blood presssure. The main goal in trying to develop a second-generation anti-hypertensive agent was to increase the duration of action so that the drug only needed to be administered once a day. The screening tests indicated that the furan ring in prazosin was particularly beneficial in this regard, and so the Pfizer scientists decided to look for an alternative oxygen heterocycle. They knew that compounds such as piperoxan (11) containing a benzodioxan group were also alpha-blockers. So Simon Campbell and his colleagues decided to investigate the effect of incorporating this group.
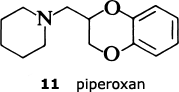
The result was doxazosin (12), a drug which only needs to be administered once a day and which, having passed through all the necessary toxicological evaluation and clinical trials, is now marketed as an anti-hypertensive agent.
We hope that this introductory video has demonstrated that research in the pharmaceutical industry is a complex and multidisciplinary process, and that progress is more often made by gradual advances rather than by sudden breakthroughs. There is no guarantee of success and thousands of different compounds may be examined in the search for the few that have the desired pharmaceutical activity. But above all, we hope it has shown the central role of organic chemistry, and organic synthesis in particular, in the discovery of pharmaceutically active compounds, and that while serendipity, or chance, often plays a part, the development of a successful new drug is increasingly the result of rational design.
 | |||
|---|---|---|---|
| Compound No. | R | Binding affinity/nmol l−1* | Effectiveness at reducing blood pressure in rats† |
| 3 | 190 | ||
| 4 | 33 | ||
| 5 | 3.4 | ||
| 6 | 2.5 | ||
| 7 | 2.0 | ||
| 8 | 1.5 | ||
| 9 | 1.0 | ||
| 10 |  | 0.2 | |
Footnotes
* Binding affinities given in terms of the so-called apparent inhibition constant Ki obtained from measurements of the amount of radioactive ligand displaced from the alpha1 receptor by various concertrations of the test sample (see the J. Med. Chem. reprint, p. 56; the unit ‘M’ used in this paper means mol l−1)Footnotes
†Effectiveness at reducing blood pressure in rats; * signifies only weakly effective through to **** which signifies highly effective.Footnotes
Note that, though compound 8 is highly effective, the reduction in blood presssure is relatively short-lived, wheareas the reduction in blood pressure observed with compound 10 (prazosin) is much longer lasting.Conclusion
This free course provided an introduction to studying Science. It took you through a series of exercises designed to develop your approach to study and learning at a distance and helped to improve your confidence as an independent learner.
Acknowledgements
The content acknowledged below is Proprietary (see terms and conditions). This content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence
Grateful acknowledgement is made to the following sources for permission to reproduce material in this course:
Course image: epSos .de in Flickr made available under Creative Commons Attribution 2.0 Licence.
The content acknowledged below is Proprietary and is used under licence.
2,4-Diamino-6,7-dimethoxyquinazolines. 1. 2-[4-(1,4-Benzodioxan-2-ylcarbonyl)piperazin-1-yl] Derivatives as α1-Adrenoceptor Antagonists and Antihypertensive Agents, Simon F. Campbell,* Michael J . Davey, J. David Hardstone, Brian N. Lewis (in part), and Michael J. Palmer, Departments of Discovery Chemistry and Discovery Biology, Pfizer Central Research, Sandwich, Kent, United Kingdom. Received March 24, 1986. Published in American Chemical Society Journal.
Financial support from Pfizer Ltd for the production of this video is gratefully acknowledged.
Adaptation of works by Simone Diolaiuti [Details correct as of 1st May 2009]
Don't miss out:
If reading this text has inspired you to learn more, you may be interested in joining the millions of people who discover our free learning resources and qualifications by visiting The Open University - www.open.edu/ openlearn/ free-courses
Copyright © 2016 The Open University