The lanthanides, despite being commonly referred to as “the rare earth metals”, are in fact found in abundance in nature and have unique optical and magnetic properties that make them of great importance in medical applications.
You might not have heard much about lanthanides. Even after studying a degree in chemistry, I hadn’t heard much about them. Lanthanides, situated apart from the main chart of the periodic table and traditionally known as the “rare earth metals”, tend to be thought of as uncommon and strange elements. However, a bit of digging into their history shows that they are far from rare and are in fact, found abundantly in nature. For instance, neodymium is more abundant than gold, and thulium (which is the least common naturally occurring lanthanide) is more abundant than iodine. The attainment of their rare moniker is instead attributed to their tendency to appear “hidden” one behind another in their mineral form in nature, which made their extraction and isolation in the eighteenth century difficult.
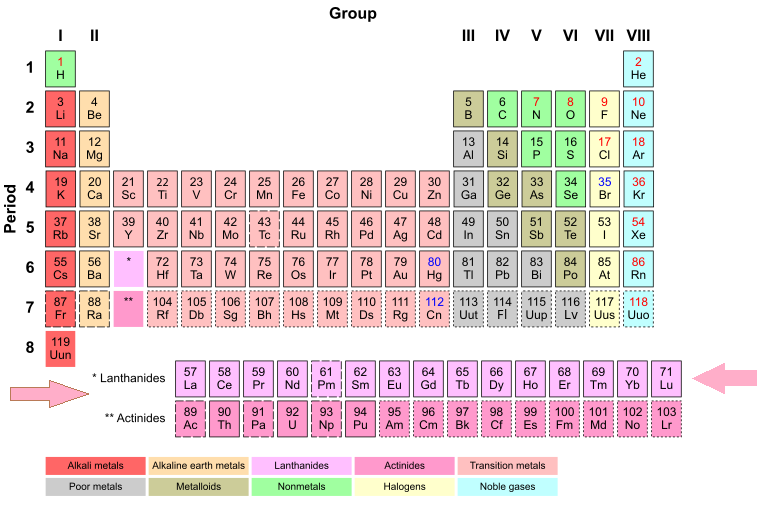 Figure 1: The lanthanides' location in the period table.
Figure 1: The lanthanides' location in the period table.
Furthermore, lanthanides are not only abundant, but they also have unique optical and magnetic properties that make them great tools in many ways, such as in medical imaging.
The lanthanides’ optical properties are due to their ability to emit fluorescent light. Fluorescence is the emission of light by atoms or molecules after having absorbed energy (usually from electromagnetic radiation or light). When lanthanides are illuminated with visible light, their electrons absorb the energy contained within the incoming photons of light, and most of this energy is, in turn, emitted in the form of light within the visible and near-infrared range of the electromagnetic spectrum. In medical imaging, lanthanide-containing molecules can be used as ‘fluorescent torches’ that aid the visualisation of cell processes, for example to indicate disease.
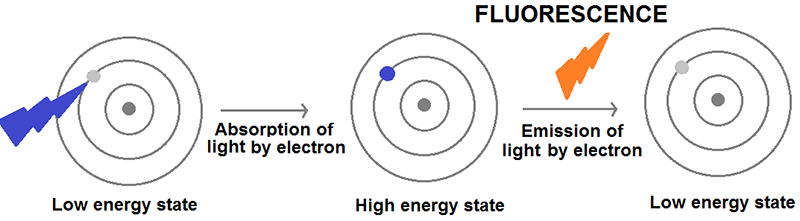 Figure 2. The process of fluorescence. When an atom or molecule is illuminated with light, it absorbs energy exciting its electrons to a higher energy state. However, electrons prefer to be in low energy states and, therefore, they get rid of this excess of energy mostly by emitting light, a process that is referred to as fluorescence.
Figure 2. The process of fluorescence. When an atom or molecule is illuminated with light, it absorbs energy exciting its electrons to a higher energy state. However, electrons prefer to be in low energy states and, therefore, they get rid of this excess of energy mostly by emitting light, a process that is referred to as fluorescence.
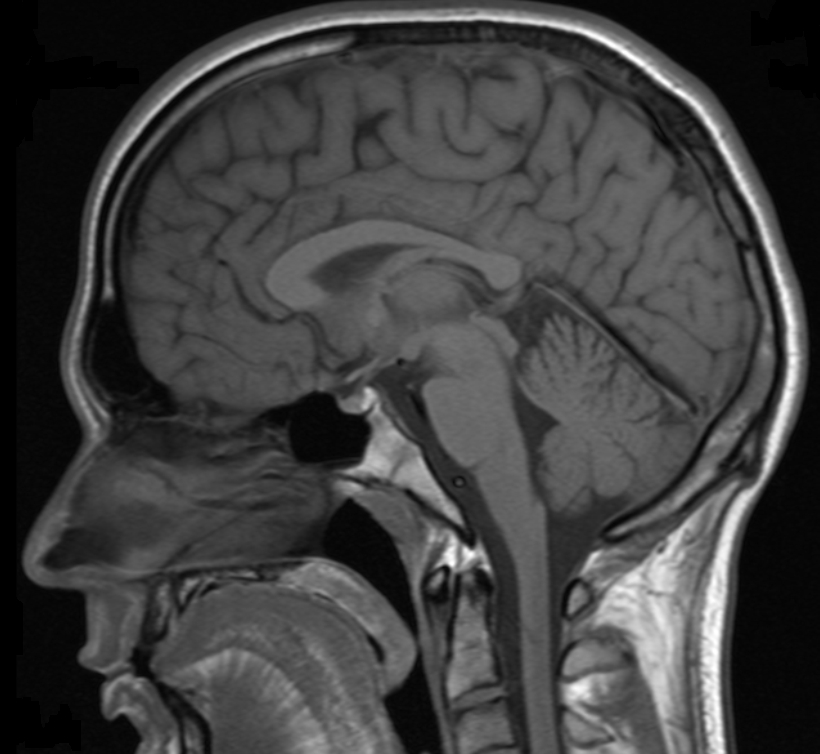
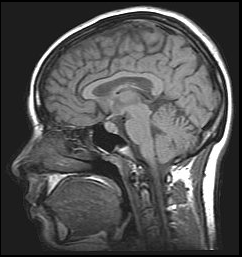 Image of the brain obtained with MRI
Another interesting feature of lanthanides is the magnetic properties of some of their ions, which makes them useful as contrast agents in Magnetic Resonance Imaging (MRI) applications. MRI is an imaging technique widely used in the clinic for the diagnosis of disease and visualisation of injuries, which utilises magnetic fields and electromagnetic radiation to create images of the physiology within the body. Contrast agents are normally needed to enhance the signal obtained from MRI and improve the quality of the images obtained, and the most popular contrast agent currently used is the lanthanide ion gadolinium(III).
Image of the brain obtained with MRI
Another interesting feature of lanthanides is the magnetic properties of some of their ions, which makes them useful as contrast agents in Magnetic Resonance Imaging (MRI) applications. MRI is an imaging technique widely used in the clinic for the diagnosis of disease and visualisation of injuries, which utilises magnetic fields and electromagnetic radiation to create images of the physiology within the body. Contrast agents are normally needed to enhance the signal obtained from MRI and improve the quality of the images obtained, and the most popular contrast agent currently used is the lanthanide ion gadolinium(III).
Therefore, further research into the properties of molecules containing lanthanides is an exciting field with the potential to lead to an easier and faster disease diagnosis process. According to Oxford Dictionary “rare” can also mean “Unusually good or remarkable”. Maybe, after all, rare wasn’t such a bad name to use to describe the lanthanides!


Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews
You know your projects stand out of the herd. There is something special about them. It seems to me all of them are really brilliant!Remove Negative Complaints and RemoveReports
julia alax - 22 September 2017 9:52am
You know your projects stand out of the herd. There is something special about them. It seems to me all of them are really brilliant![url=https://removereports.com]Remove Negative Complaints and RemoveReports[/url]