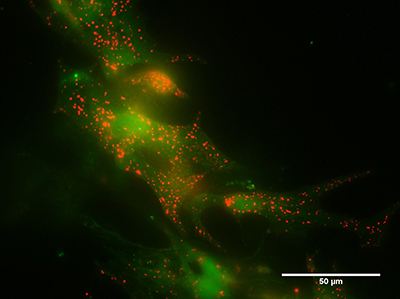
 Cardiac myocytes loaded with a fluorescent stain for lysosomes (red) and a dye measuring the intracellular calcium concentration (green). The brighter the green fluorescence, the higher the calcium concentration
The causes of heart problems in juvenile dementia, also called CLN3 (Batten) disease, are not yet fully understood and current research in the underlying reasons is limited. To learn more about this disease and its effects on the heart, we are studying cells to find changes in structure and calcium signalling inside cells.
Cardiac myocytes loaded with a fluorescent stain for lysosomes (red) and a dye measuring the intracellular calcium concentration (green). The brighter the green fluorescence, the higher the calcium concentration
The causes of heart problems in juvenile dementia, also called CLN3 (Batten) disease, are not yet fully understood and current research in the underlying reasons is limited. To learn more about this disease and its effects on the heart, we are studying cells to find changes in structure and calcium signalling inside cells.
CLN3 is a rare disease which affects the eyes, brain and heart in humans, starting with eyesight deterioration around the age of 4-8 years. During their lifetime, patients experience worsening symptoms including seizures and a dementia-like deterioration. In their 20s they often develop heart problems and end of life is around the age of 30.
The disease is caused by inheriting CLN3 gene mutations from both parents. The mutated genes cause a structural alteration in the CLN3 protein, which is normally found on lysosomes. These are small structures found inside cells, they are enclosed by a membrane, carry enzymes and store calcium. Calcium is an important ion in cells, responsible for signalling in a range of events, such as muscle contraction.
Lysosomes are also involved in a process known as ‘autophagy,’ where small pockets of membrane form around waste material, forming ‘autophagosomes.’ Lysosomes then fuse with autophagosomes to form ‘autolysosomes’ and the enzymes carried by the lysosomes digest the waste to clear it from the cell.
In CLN3 disease, autophagy is altered and waste builds up inside the cells, so CLN3 is known as a ‘lysosomal storage disorder.’ The exact function of the CLN3 protein in a cell is currently unknown and it is also not clear yet how the build-up of material in the cells is linked to CLN3 disease, so these are important questions for research.
It has recently been discovered that lysosomes store calcium. Release of calcium from stores in the cell is responsible for many processes; for example, calcium release in heart muscle cells cause them to contract and ‘beat’ in time with the electrical impulse which paces the heart. When this calcium release is altered, the cells may not stay in rhythm, causing abnormalities in the heart beat and potentially serious medical conditions.
To investigate if heart cells with the mutated form of CLN3 have different structure and function compared to ‘normal’ heart cells we are using fluorescent dyes to visualise cellular components such as lysosomes and to measure changes in the calcium concentration. This will help us to understand how the role of the CLN3 protein in the cell and how it the mutations cause such a tragic disease.


Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews