2.3.3 Receptors with intrinsic enzymatic activity
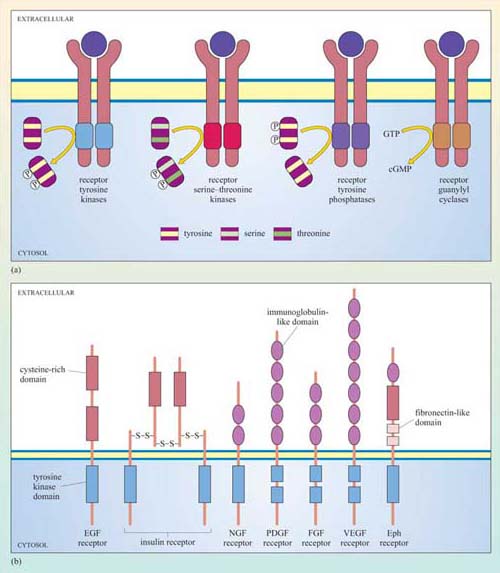
Receptors with intrinsic enzymatic activity are the second biggest group of receptors after the GPCRs. They include four types according to the form of enzymatic activity of the intracellular domain (Figure 23a).
Receptor tyrosine kinases (RTKs) On activation, the kinase domain phosphorylates tyrosine amino acid residues. There are seven classes of RTK with different extracellular domains (Figure 23b).
Receptor serine–threonine kinases On activation, the kinase domain phosphorylates serine and/or threonine amino acid residues.
Receptor tyrosine phosphatases The intrinsic tyrosine phosphatase activity of the enzymatic domain is suppressed on activation.
Receptor guanylyl cyclases The enzymatic domain generates the second messenger cGMP from GTP following activation.
The basic model of activation for receptors with intrinsic enzymatic activity is that ligand binding induces dimerization (in some cases oligomerization) of the receptor, which brings together the cytoplasmic enzymatic domains and leads to a change in enzymatic activity. Dimerization may occur between different receptors that bind the same ligand (heterodimerization), or between the same type of receptor chains (homodimerization), or either. RTKs, RTPs and guanylyl cyclase receptors generally form homodimers (an exception being the epidermal growth factor (EGF) receptor tyrosine kinase), whereas receptor serine–threonine kinases generally form heterodimers. In some cases, oligomerization of several receptors is required for activation.
We shall now describe the general mechanism of activation of RTKs in more detail. There are several strategies by which an extracellular signal may achieve RTK dimerization leading to activation of the receptor:
Ligands such as EGF, which is a monomer, have two binding sites for each receptor unit.
Platelet-derived growth factor (PDGF) is a covalently linked dimer, in which one subunit binds to one PDGF receptor chain, and the other subunit binds to another PDGF receptor chain (Figure 24).
Fibroblast growth factor (FGF) binds to proteoglycans (located on the cell surface or on the extracellular matrix) and induces clustering of FGF receptors.
Ephrins are bound to the plasma membrane of the signalling cell in clusters, and thereby induce association of their receptors (called Eph receptors) on the target cells following cell–cell contact.
The insulin receptor is a tetramer prior to binding insulin: on insulin binding, activation occurs by rearrangement of the different receptor chains that brings the kinase domains in close proximity.
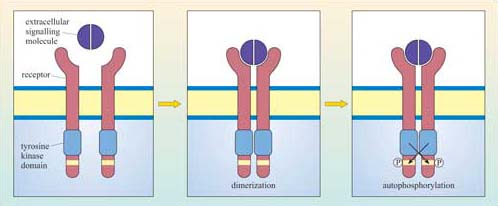
Although there can be a great deal of variation in the extracellular domains of RTKs (Figure 23b) and in the ways the extracellular signal binds to its receptor, the basic mechanism of receptor activation still applies (Figure 24). Association between receptors results in cross-phosphorylation of the kinase domain on each intracellular tail of the RTK, a process called autophosphorylation. This results in an increase in its intrinsic kinase activity, which causes phosphorylation of tyrosines in other parts of the cytoplasmic domain (and/or other proteins). Autophosphorylation generates docking sites on the receptor for downstream signalling proteins that contain SH2 domains.
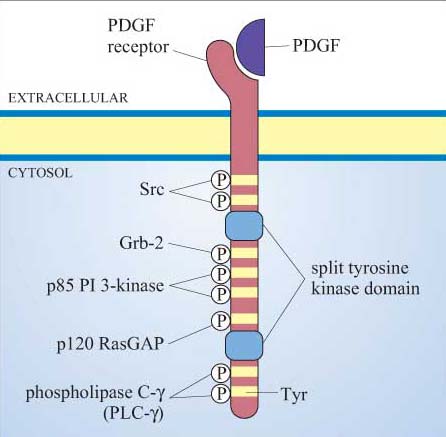
Many proteins can bind to phosphotyrosine (pY) residues, but these interactions are influenced by nearby amino acid side-chains (see previous section). For example, the PDGF receptor has specific phosphotyrosine sites, which can bind the regulatory (p85) subunit of phosphatidylinositol 3-kinase (PI 3-kinase), a GTPase-activating protein (p120 RasGAP) and phospholipase C-g (PLC-γ), among others (Figure 25). The insulin receptor extends its docking potential by associating with a large protein, insulin receptor substrate 1 (IRS-1), which has many tyrosine residues that can be phosphorylated by the insulin receptor (Section 4). These proteins are called ‘docking proteins’ and may be activated by being directly phosphorylated by the RTK, or by interactions with other docking proteins or plasma membrane molecules. Some docking proteins are adaptor proteins that merely serve to bring other signalling molecules into place. The overall effect of this system is the recruitment of many different signalling pathways, allowing the modulation of many cellular processes.