3.4.1 Calcium ions
The Ca2+ concentration is normally low in the cytosol (~10–7 mol 1–1) compared with the extracellular space (~10–3 mol 1−1). There are several mechanisms for achieving this. The most widespread are ATP-dependent Ca2+ efflux pumps on the plasma membrane, which pump Ca2+ ions out of the cell. Muscle and nerve cells, where oscillations in intracellular Ca2+ concentration often occur, employ an additional Na+-driven Ca2+ exchanger. There are also pumps driving cytosolic Ca2+ into the endoplasmic reticulum, so that it acts as a Ca2+ store. When Ca2+ channels are transiently opened by a signalling protein, Ca2+ floods down the concentration gradient into the cytosol. The result is a rapid 10–20-fold increase in the cytosolic Ca2+ concentration in the cytosol, which in turn activates numerous different Ca2+-dependent proteins, such as PKC.
There are three main types of Ca2+ channel:
a.IP3-gated Ca2+channels on the ER (Section 3.3).
b.Voltage-dependent Ca2+channels in the plasma membrane, which open when the membrane is depolarized. These are used, for example, at neuron terminals, where Ca2+ release stimulates secretion of neurotransmitters.
c.Ryanodine receptors closely associated with receptors on the plasma membrane, which respond to changes in membrane potential, and release Ca2+ from the sarcoplasmic reticulum in skeletal muscle cells (as described in Section 1.5, Figure 7) or from the ER in neurons. Their name derives from their sensitivity to the plant alkaloid ryanodine.
Ca2+-sensitive fluorescent indicators can be used to monitor changes in intracellular Ca2+. After stimulation with an extracellular signal, opening of channels and release of Ca2+ in the cytosol by the mechanisms described above result in local increases in Ca2+ concentration, often circumscribed to small regions of the cell. These increases usually reflect the opening of individual channels or of small groups of channels; these changes in intracellular Ca2+ are called ‘quarks’ or ‘blips’. If the signal is persistent and sufficiently strong, the change in the concentration of Ca2+ spreads across the cell. Under the fluorescent microscope, it appears as if an initial wave of high Ca2+ is followed by other waves propagating through the cytosol, with Ca2+ concentrations first rising and then returning to basal levels; these changes are referred to as ‘spikes’ or ‘oscillations’, and can be repeated at intervals of seconds or minutes. The frequency of Ca2+ oscillations can determine the response. For example, a low frequency of Ca2+ spikes may trigger transcription of one set of genes, whereas a higher frequency triggers transcription of a different set. The sensitivity of the cellular response to the frequency of Ca2+ oscillations requires a special kind of protein called calmodulin.
Calmodulin is abundant, constituting about 1% of total cellular protein. It has no intrinsic catalytic activity, but on binding to Ca2+ it is able to modulate the activity of other proteins. It has four Ca2+ binding sites (small, helical Ca2+-binding motifs called ‘EF hands’, which are also present in some other Ca2+-binding proteins); at least two of them must be occupied for it to adopt its active conformation (Figure 33).
What term describes the regulation of calmodulin by Ca2+?
Calmodulin is allosterically regulated by Ca2+ ions
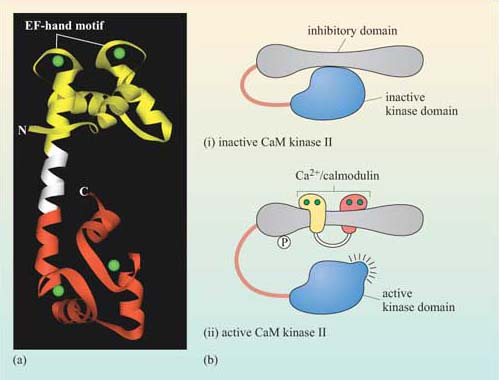
The biggest class of proteins affected by calmodulin is called the Ca2+/calmodulin-dependent protein kinases (CaM kinases), which are serine–threonine kinases. CaM kinase II is found in high quantities in the brain, particularly at synapses. It seems to be involved in some kinds of memory, since mice with mutations in CaM kinase II have specific learning difficulties. On binding Ca2+/calmodulin, CaM kinase II activates itself by autophosphorylation, the degree of activation being dependent on the oscillation frequency of Ca2+ concentration.
Many members of the protein kinase C family are Ca2+-binding proteins, but in these proteins Ca2+ binds to a larger domain, known as the ‘C2 domain’. Ca2+ binding changes the net charge of the C2 domain, enabling it to bind negatively charged phospholipids such as DAG in the plasma membrane (see Section 1.4.2 and Figure 31). Here, Ca2+ is acting as a switch, helping to change the localization of the enzyme.