3.6.1 The MAP kinase pathway
The MAP kinase pathway is so called because the last component of the pathway was originally identified as a kinase activity in EGF-stimulated cells – hence the name ‘mitogen-activated protein kinase’ (MAP kinase), as it stimulates cell growth and proliferation.
What is the mechanism of activation of the EGF receptor?
The EGF receptor is an RTK (Figure 23b), which becomes activated by dimerization. By contrast with most other RTKs, the EGF receptor does not form homodimers.
It was subsequently found that MAP kinase acts downstream of Ras, and that there are also two intermediary kinases (Raf and MEK in mammals), making it a three-kinase signalling module (Figure 36). MAP kinase forms the link between Ras in the plasma membrane and downstream effectors such as transcription factors in the nucleus. As such, it is crucial to signalling from growth factor receptors and other RTKs (and tyrosine kinase-associated receptors).
When Ras is activated, it recruits the serine–threonine kinase Raf to the membrane, where it becomes activated in a poorly understood mechanism involving membrane-bound kinases other than Ras itself (see Box 3). Raf is the first of the three kinases in the MAP kinase cascade, and is therefore also known as MAP kinase kinase kinase (abbreviated to MAPKKK). Raf phosphorylates (and thereby activates) the next kinase (a MAP kinase kinase, or MAPKK), which in mammalian cells is called MEK (MAP/ERK kinase). In turn, this phosphorylates MAP kinases (the classic one in mammals being ERK, e xtracellular signal r egulated k inase). In their active state, MAP kinases are able to translocate to the nucleus and are able to phosphorylate numerous nuclear target proteins, leading to such major cellular events as proliferation or differentiation.
Why is there a chain of three kinases rather than just one? This is partially explained by the following principles.
Firstly, we have to consider signal specificity. MAP kinases are very active proteins with many targets, and so need to be under close regulation. MEK provides this, since the peculiar phosphorylation requirements of MAP kinases (see below) mean that MEK is the only known activator of MAP kinases. Assembling the three-kinase signalling module on a scaffold, as happens in some MAP kinase pathway situations, can also help maintain specificity.
Secondly, a sequence of kinases gives an opportunity for signal amplification.
Thirdly, signal duration can radically change the signalling outcome (Section 3.8). Indeed, RTKs and Ras are generally inactivated fairly quickly by tyrosine phosphatases and GAPs, respectively, whereas the MAP kinase cascade can remain active for more extended periods of time (Section 3.7).
Finally, the signal needs to be translocated from one part of the cell (the plasma membrane) to another (the nucleus). The MAP kinase pathway does not employ any second messenger molecules to broadcast the signal, but MAP kinase itself is the nearest protein equivalent, translocating to the nucleus once activated.
Box 3 Investigating the activity of signalling molecules
The activity of signalling molecules can be altered by site-directed mutagenes is which has proved very useful in the engineering of constitutively active or inactive versions of kinases. The inactive versions can act in a dominant negative manner within a cell, blocking the downstream pathway, whereas the constitutively active versions can permanently switch on the downstream pathway. These can be very useful tools for investigating signal transduction pathways. The example we shall examine is the mutagenesis of MEK, which is activated by phosphorylation on two nearby serines, Ser 217 and Ser 221, within the activation loop. The experiment, whose results are illustrated in Figure 38, shows the consequences of introducing MEK constructs that have been mutated at these residues by site-directed mutagenesis into cells. The effects of these mutations are assessed by determining the activity of its immediate downstream effector, ERK, by an in vitro kinase assay.
Replacing serine residues with glutamate residues at positions 217 and 221 creates a constitutively active mutant of MEK, which continuously activates ERK.
From your knowledge of amino acid structure, suggest why glutamate instead of serine at positions 217 and 221 creates a constitutively active mutant.
Glutamate is negatively charged, so to some extent it mimics phosphoserine.
Replacing either of these two key serines with alanine creates an interfering mutant of MEK, which blocks the activation of ERK.
Again, suggest why replacing serine with alanine stops MEK functioning normally.
Because the alanine side-chain has no –OH group, it cannot be phosphorylated, so MEK cannot become activated and cannot phosphorylate ERK.
The technique used to introduce the mutant MEK constructs into cells is known as ‘transfection’ (Figure 39a), a technique for which there are several variants. The principle is to induce tissue culture cells to take up a plasmid expression vector containing the gene to be expressed. Cationic lipids are used to promote uptake of plasmid DNA. In this experiment, a plasmid was used in which the mutant MEK gene was downstream of a promoter (SV40 in this instance). This protocol is suitable for experiments that terminate within a day or two (called ‘transient’ transfection). When a cell line that permanently expresses the gene is needed, the plasmid must be maintained and replicated within the cells.
In this case, the expression vector must have the appropriate replication sequences and a selectable marker (such as the resistance gene to an antibiotic like puromycin), so that transfected cells may be selected from the untransfected neighbours; this is known as ‘stable transfection’ (Figure 39b). When short-term effects need to be observed in individual cells, or where cells are difficult to transfect, recombinant DNA (or protein) may be microinjected (Figure 39c).
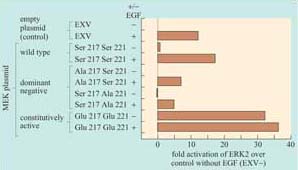
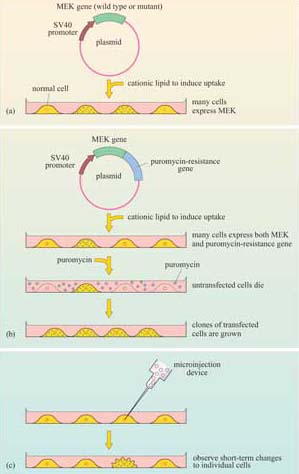
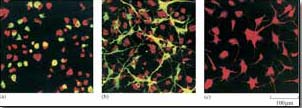
Figure 40 shows the rat PC12 neural cell line microinjected with MEK mutant DNA. The constitutively active MEK mutant induces differentiation of this cell line (as seen by the outgrowth of neural processes, also known as ‘neurites’), similar to that induced by nerve growth factor (NGF). This evidence supports a role for the ERK MAP kinase pathway in neural outgrowth in this cell type.
The activity of a signalling protein can be altered not only by changing the catalytic activity of its kinase domain, but also by translocating the signalling protein to particular subcellular localizations, which can be critical for their activation and/or ability to access their targets. This can be investigated experimentally by ‘forcing’ the protein of interest into a particular subcellular localization. The principle has been successfully exploited, for example, to investigate the activation of Raf. The membrane localization signal of K-Ras (the amino acid sequence Cys–Val–Ile–Met), which is post-translationally prenylated by addition of a farnesyl residue, was added to the carbon terminus of Raf. This modification constitutively localized Raf to the membrane, resulting in its constitutive activation. The activated Raf could be further activated by EGF independently of Ras, which suggested that Ras acts as a temporary membrane anchor for Raf (as shown in Figure 36), and that Raf is also activated by other membrane-associated signals when in the membrane environment.
Proteins may be targeted to a number of cellular locations by this technique. For example, nuclear localization sequences can be added to signalling proteins (such as MAP kinases) to investigate their role in gene transcription.
What signal sequence do you think would target a protein to the nucleus?
A nuclear localization signal (NLS).
Activation of MAP kinases involves specific phosphorylation of residues within the activation loop of the catalytic domain of the protein, For example, Raf activates MEK by phosphorylating two specific serines within the activation loop (Box 3). The activation requirements of MAP kinase are even more specific. It requires activation by phosphorylation of a threonine residue and a tyrosine residue. These two are separated from the threonine receptor by a single amino acid within the activation loop. In ERK this motif is Thr–Glu–Tyr, in which the threonine and tyrosine residues can be phosphorylated only by MEK, because MEK is a member of the very unusual dual-specificity kinases, which are able to phosphorylate serine–threonine and tyrosine residues.
Activated MAP kinase family members are activators of immediate ‘early genes’, so called because they are activated within minutes of cell stimulation. Many of these genes encode transcription factors (Section 3.8), which then switch on other sets of genes, thereby initiating cellular programs of differentiation or proliferation. There are also cytosolic targets of MAP kinases, which include regulators of protein synthesis. As an example, MAP kinase phosphorylates another kinase called Mnk1, which, in turn, phosphorylates and activates the translation initiation factor 4E (eIF-4E.
There are several MAP kinase pathways. The pathways employing ERK represent the classic pathway, but there are also two other major mammalian MAP kinase pathways implicated in cellular responses to stress (not discussed here). Moreover, the MAP kinase pathway is conserved across the animal kingdom and even in yeast and plants, as shown in Table 3. In particular, the model organisms C. elegans and Drosophila have provided extremely useful experimental systems for investigating MAP kinase pathways, and for showing that it operates downstream of growth factor receptors and Ras.
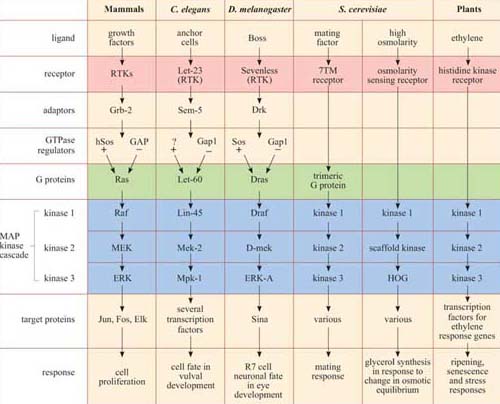
*There is remarkable conservation of MAP kinase signalling pathways, from mammals to plants. In addition to the MAP kinase cascade (blue), there are other striking homologies in several of the pathways, especially between growth factor pathways in mammals, vulval induction in nematodes and eye development in fruit-flies, where the receptors (pink), adaptors, GTPase regulators and G protein homologues (green) are also related. Not all the names of all the signalling proteins here are explained, and they need not be remembered; they are included merely to illustrate the conservation of the pathways. The GEF for the Ras homologue in C. elegans remains unidentified, and is denoted as ‘?’.