3.7 Protein phosphatases
Together with inositolphospholipid phosphatases, protein phosphatases are key regulators of signal transduction pathways. Like protein kinases, protein phosphatases are either tyrosine phosphatases (the majority of protein phosphatases, some of which are shown in Figure 44) or serine–threonine phosphatases (including the phosphoprotein phosphatase family, designated PP1–6), which will be described in Section 4, or, rarely, dual-specificity phosphatases.
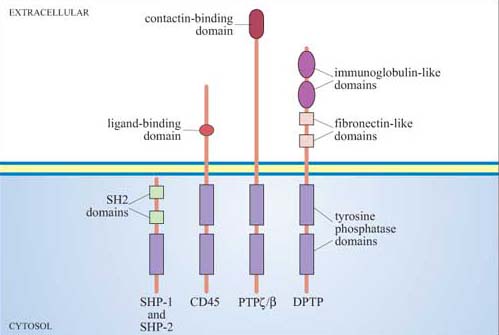
Phosphatases are required to inactivate signalling proteins that have been activated by phosphorylation. Many tyrosine phosphatases such as SHP-1 and -2 have SH2 domains, and are recruited to the membrane following ligand-stimulated phosphorylation of receptors. For example, the tyrosine phosphatase SHP-1 binds to phosphotyrosines on activated cytokine receptors such as the erythropoietin (Epo) receptor, and is then phosphorylated by JAK2, which activates it. Active SHP-1 can downregulate (damp down) the JAK/STAT signalling pathway by dephosphorylating specific JAKs and STATs. It is therefore acting as a negative regulator.
Another role for phosphatases occurs when they activate a protein that is held in an inactive state by phosphorylation.
You have already met an example of a protein regulated in this way. What is it?
Src, which in its inactive state has an inhibitory phosphate on Tyr 527 (Section 1.6).
Lck is a Src family tyrosine kinase (Figure 26), which is dephosphorylated and thereby activated by the membrane-bound tyrosine phosphatase CD45. (CD45 plays an essential role in the activation of leukocytes following antigen presentation.) CD45, like many of the PTPs, is a transmembrane protein (Figure 44); such proteins are referred to as receptor tyrosine phosphatases.
One of the most well-studied phosphatases is the dual-specificity phosphatase MKP-1, which inactivates MAP kinase. The MKP-1 gene is one of the immediate early genes expressed following MAP kinase activation, being expressed approximately 20 minutes after cell stimulation. As MKP-1 levels rise, MAP kinase is dephosphorylated and inactivated.
What type of regulation is effected by MKP-1 on MAP kinase?
Feedback inhibition.
Because negative feedback by MKP-1 is a transcription-dependent mechanism, it helps to explain the relatively long duration of MAP kinase activation.