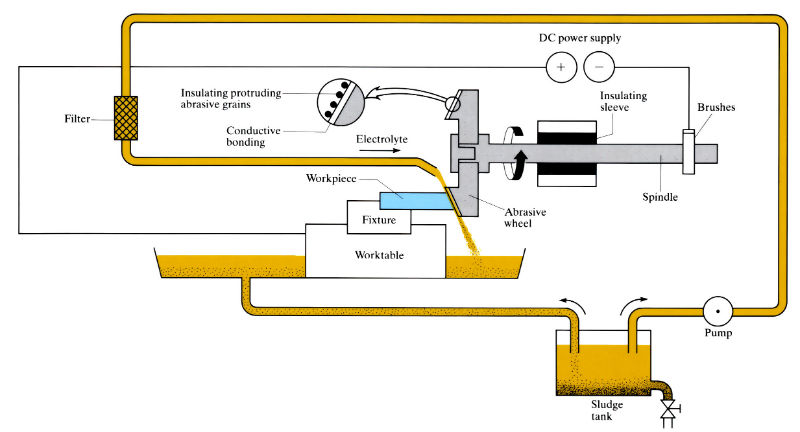
Manufacture:
- Wheels are a conductive binder of brass, bronze or copper containing non–conductive alumina or diamond abrasive grit particles. Grit size, which determines the work gap, is typically 0.01–0.25 mm
- Although the wheels are more expensive than those for conventional grinding, overall wheel costs are only 1/10 as much, as only 2–5% of the wheel removed by abrasion.
- Low wheel wear means less frequent wheel dressing, although the longer runs, between dressing, necessitate more precise wheel truing than for conventional grinding; typically ±0.005–0.013 mm.
- Despite high wheel costs, and capital costs 5–10 times that of conventional grinding machines, the high ratio of stock removal/wheel wear and the longer production runs, due to less frequent wheel dressing, lead to 25–40% reduction in tool grinding costs.
- Wheels must be “conditioned” before use by reversing the electrical leads for a short time. This removes a little of the metal binder, causing the abrasive particles to protrude.
- Although NaCl and KNO3 sometimes used, electrolyte is usually NaNO3 (120–240 g l–1) at 25-40°C and 15–70 kPa pressure. As the electrolyte is corrosive, machine parts must be corrosion resistant and workpiece must be thoroughly cleaned after grinding. High throughput is desirable since the corrosive electrolyte means machines cannot be shut down for long without electrolyte removal and thorough cleaning.
- Maximum wheel contact arc length should not exceed 20–25 mm to avoid electrolyte boiling. If necessary to exceed this value, electrolyte must be supplied to the grinding area by auxiliary methods, or feed rate must be reduced.
- Feed rates depend on the grinding mode: surface, plunge, cylindrical, traverse or form grinding. Typical feed rates: surface grinding, 25 mm min–1 with 0.25 mm depth of cut at 155 A cm–2; plunge grinding 1.25 mm min–1 at 80 A cm–2.
- Typical operating conditions are 50–3000 A at 4–16 V.
- Metal removal rates are proportional to current density, limited by the anodic dissolution rate for a particular alloy–electrolyte system and the boiling point of electrolyte. Removal rates are 5–10 times those of conventional grinding for hard materials; typically 0.15 cm3 min–1 (100 A)–1.
- Main use of process is the shaping and sharpening of WC cutting tools, although it has been used for sharpening hypodermic needles and machining thin, fragile, honeycombed structures.
Materials:
- Almost any conducting material, irrespective of hardness, but best suited to hard materials (>400Hv) and the difficult-to-machine materials such as zirconium and beryllium.
- Dissimilar combinations of materials can be ground as long as they are electrically conductive.
- Due to the low machine forces (0.5–1 MPa) the material is stress free and with the minimum of distortion.
- Unlike conventional grinding there is no work hardening of the surface of the material.
- Poor operating conditions can lead to hydrogen pick-up in steels.
Design:
- Low machining forces (0.5–1 MPa) make the process ideal for thin fragile workpieces.
- No burring of workpiece.
- Surface texture similar to that of a metallographic polish.
- Surface finish improves with current density and feed rate, 0.2–0.8 µm Ra is typical, although 0.025–0.1 µm is possible with careful control of operating conditions.
- Outside corner radii of 0.025 mm are possible, although inner corner radii are limited to 0.25–0.4 mm due to “overcut” during electrochemical reaction.
- Accuracies are typically ±0.025 mm on a one-pass cut, but ±0.005 mm can be achieved on the final pass without the use of electrolyte.
See Also: Electrochemical machining and Grinding
This article is a part of Manupedia, a collection of information about some of the processes used to convert materials into useful objects.
Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews