Nickel-phosphorus (8–12% P) is deposited to give hardness of 500–600 VPN (as deposited) and 800–1000 VPN (heat treated 400⁰C for 60 min). Gives more uniform deposit than electroplating, and better corrosion resistance. Surface has good lubricating properties due to phosphorus content. Deposit thickness 75 µm.
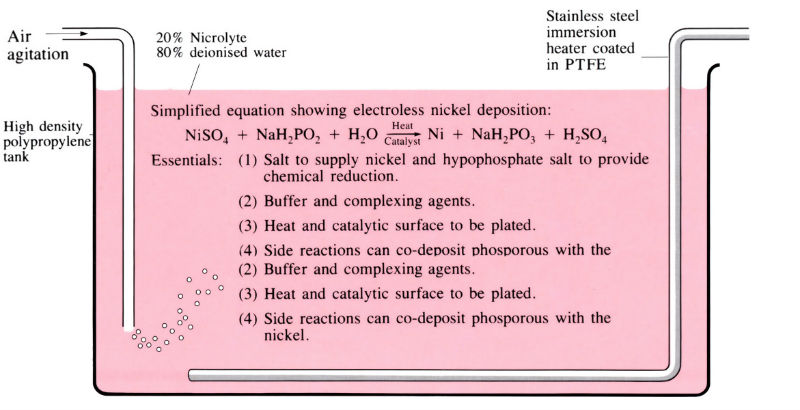
Composition of nicrolyte
30 g l-1 nickel sulphate (NiSO4.6H2O) – source of nickel ions (5–7 g l-1). 20 g l-1 sodium hypophosphite (NaH2PO2. H2O) – reducing agent, source of energy for process. (Sulphuric acid and ammonium hydroxide added to control pH to 4.5–5.0).
Manufacture:
- Production line consists of the following stages:
- alkaline soak or electroclean
- cold or warm water rinse
- acid pickle
- rinse
- electroless plate
- Additional pre-treatment is needed to produce a catalytic surface (Pd, Rh, Ag, Au, Ni, Fe, Co, Ru, Al) for plating copper, silver and plastics.
- Process does not require sophisticated equipment, but requires good process control of the following variables: temperature (70–95°C), pH 4.5–5.0, continuous filtration and post-heat treatment.
- Barrel plating is used for small components.
- Composites can be incorporated into the layer.
- Production costs compared with hard chrome plating: electroless nickel £0.5 (0.0065 cm)-1 cm-2, hard chrome £0.2 (0.0065 cm)-1 cm–2.
- Gives more uniform deposits than electroplating.
- Operating parameters:
- nickel content of bath 57 g l-1
- solution temperature 70–95°C
- solution pH 4.5–5.0
- bath loading 61–245 cm2 l-1
- rate of deposition 20 μm h-1
- Optimum balance of bath loading can affect losses or bath solution constituents, especially sodium hypophosphate.
- Coatings can be post-heat treated to increase deposit hardness up to 1000 VPN (1 h at 400°C).
Materials:
- A wide range of materials can be coated; plated directly – steels, cast iron, Al, Co, stainless steels, Ti and Mg; requiring catalytic initiation – Cu, Ag and plastics.
- Surfaces which cannot be plated directly are Zn, Cd and Pb.
- Materials which can form coatings include the following:
- Ni-(8–12%)P
- Ni-B
- Ni-Co
- electroless Ni composite coatings (25–30% by volume) with coating thicknesses of 10–25 μm
- electroless Ni-SiC (7 µm size)
- electroless NiAl2O3 (4–5 µm size)
- electroless Ni-polycrystalline diamond (6 µm size)
- electroless Ni-PTFE
- electroless nickel with the inclusion of 20–30% by volume of 6 µm polycrystalline diamond has given the best improvement in wear rate tests
| Material | Wear rate (µm h-1) |
|
Electroless Ni-P as-plated |
23,000 |
|
Electrodes Ni-P heat treated |
2000 |
|
Electroless Ni-SiC |
300 |
|
Electroless Ni-Al2O3 |
110 |
| Electroless Ni-polycrystalline diamond |
5 |
Design:
- Main advantage of electroless plating is that uniform deposits can be achieved, unlike electroplating which is uneven.
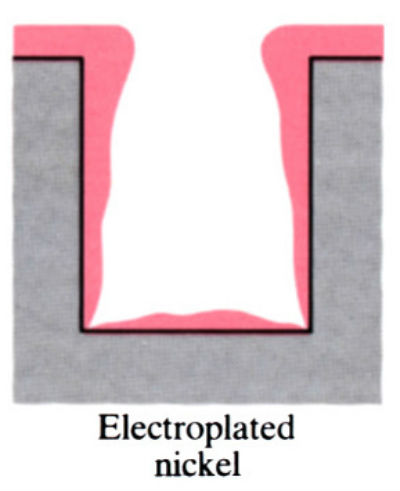
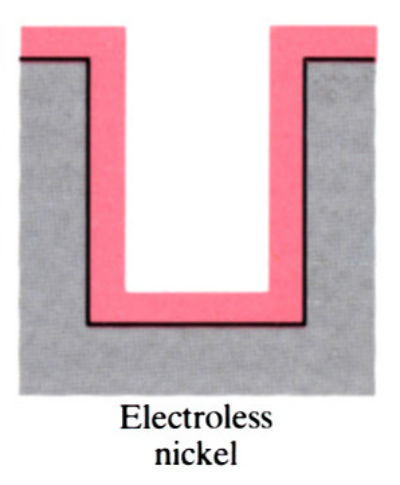
- Electroless coatings will penetrate into narrow through and blind holes, provided these are positioned so that there is no gas entrapment.
- No complex jigging and anode arrangements are required.
- Can be used for a wide range of engineering applications where chemical resistance, high hardness and resistance to wear and abrasion are required.
- Applications include:
- plastic injection moulds
- die casting moulds
- glass production moulds
- salvage of worn parts
- gears, bearings, crankshafts, pumps, hydraulic cylinders, ball studs and transmission thrust washers.
- Tolerances on deposit thickness range from 0.25 to 2 µm
- Competes directly with hard chrome-plating.
See Also: Electroplating, Chemical vapour deposition (CVD), Physical vapour deposition (PVD), Thermal spraying, Ion implantation and Plasma nitriding/carburising.
This article is a part of Manupedia, a collection of information about some of the processes used to convert materials into useful objects.
Rate and Review
Rate this article
Review this article
Log into OpenLearn to leave reviews and join in the conversation.
Article reviews