3.1 Signalling proteins act as molecular switches
One of the most important aspects of how proteins function in signalling pathways is that they can act as molecular switches. Most signalling proteins exist in interchangeable active or inactive states. They can therefore behave as rapid molecular switches, being either ‘on’ or ‘off’. Such acute changes in activity are essential for the transmission of signals within cells.
Altered protein expression often accompanies activation of cellular communication for minutes or hours, but changes in protein levels are usually not sufficiently rapid to convey dynamic signals. Rather, the activity of signalling proteins is acutely modulated over a much shorter time frame (seconds to minutes). The most rapid responses are seen with ion channels, where the binding of a ligand causes the channels to open within fractions of a second. The rapid activation of ion channels is critical for the transmission of electrical signals in neurons and muscle cells.
How does a protein actually convey a signal? The answer lies in the conformation of the protein, which is related to its activity. Usually, the upstream signal induces a change in a protein’s conformation, which enables it to carry out its downstream signalling function. Common ways of modulating a protein’s activity are by covalent modification and allosteric regulation.
One of the most commonly encountered forms of covalent protein modification in signalling pathways is phosphorylation. Protein kinases catalyse the transfer of the terminal (γ) phosphate of ATP to a tyrosine, serine or threonine residue on their target protein. Kinases are commonly named in relation to their upstream activator or downstream substrate protein, but are also noted by the amino acids that they phosphorylate. An example of a kinase that you will encounter later in this course is MAP kinase, which is a serine/threonine kinase (often abbreviated to Ser/Thr kinase). You should remember that Ser/Thr kinases phosphorylate serine and/or threonine residues, tyrosine kinases phosphorylate tyrosine residues, whereas dual-specificity kinases can phosphorylate serine, threonine and tyrosine residues. Some kinases actually phosphorylate themselves. This is known as autophosphorylation, and can be critical for kinases to become fully active.
The length of time that a signalling protein remains in its phosphorylated state before being dephosphorylated can be important in determining the signalling outcome. If phosphorylation induces activation, the longer a signalling protein is active, the more downstream signalling molecules it can activate or generate. Many phosphorylated signalling proteins are protein kinases themselves, whose activation results in a series of phosphorylation cascades.
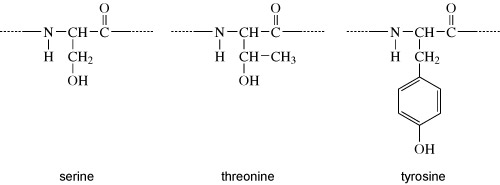
Activity 6 Chemical structures
Proteins can be phosphorylated on serine, threonine or tyrosine residues. The chemical structures of the side chains of these amino acids are shown in Figure 7. What do these all have in common?
Answer
They all have a hydroxyl group (OH). It is at this hydroxyl group that the phosphate group is added, as shown in Figure 8.
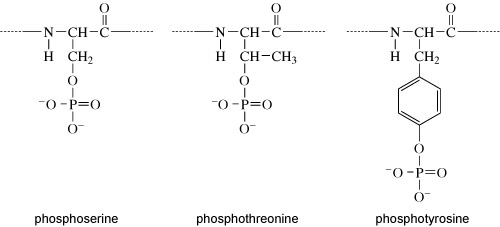
Kinases recognise specific binding sites on their substrate proteins and typically only phosphorylate amino acids within a particular sequence, called a consensus sequence. How does phosphorylation of a protein affect its activity? Addition of a phosphate group can change the conformation of a protein, or alter the interactions of a protein with substrates or other molecules. Some proteins have binding sites for specific phosphorylated motifs on other proteins. Phosphorylation can therefore facilitate the association of proteins. Over a longer timescale, phosphorylation may be a signal for protein degradation, or translocation of a protein to another part of the cell. A phosphate group is removed from a protein by a phosphatase enzyme, generating inorganic phosphate and returning the protein to its unphosphorylated form (Figure 9(a)).
There are numerous different protein kinases in eukaryotic cells. They phosphorylate specific substrates by recognising consensus sequences. Though less numerous than kinases, there are also many phosphatases in eukaryotic cells. Some phosphatases are highly substrate-specific, acting on only one or two phosphorylated proteins, but there are others that can act on a broad range of substrates.
Allosteric regulation of signalling proteins occurs when they bind to molecules including other proteins, lipids or small non-protein ligands including calcium, cyclic AMP and guanosine nucleotides (e.g. GTP). The binding of these allosteric regulators is not random, but is conveyed by specific domains (regions associated with particular functions) within proteins. The binding of an allosteric regulator changes the conformation of its target protein, thereby causing a switch in activity.
Many signalling pathways include guanine nucleotide-binding proteins (G-proteins). These proteins can bind both GDP and GTP, but are only active when they have a GTP molecule bound (Figure 9(b)). There are two classes of G-proteins – trimeric and monomeric. As their names suggest, trimeric G-proteins are composed of three subunits (α, β and γ). Monomeric G-proteins consist of only a single polypeptide that is analogous to the α subunit of trimeric G-proteins.
Trimeric G-proteins interact with a class of receptor known as G-protein-coupled receptors (GPCRs). In their inactive states, both trimeric and monomeric G-proteins have GDP bound. The activation of G-proteins involves the exchange of GDP for GTP. This exchange is promoted by an upstream signalling protein called a guanine nucleotide-exchange factor (GEF). In the case of trimeric G-proteins, the upstream GEF would be a GPCR. Monomeric G-proteins are activated by a range of GEFs that participate in many different signalling pathways. When G-proteins bind GTP they adopt a conformation in which they are active.
G-proteins have the intrinsic ability to hydrolyse GTP to GDP. This means that they are only active for a limited time, because when the bound GTP becomes hydrolysed to GDP they will return to an inactive state. The GTPase activity of G-proteins can be accelerated when they bind to their downstream targets and also by association with regulatory proteins known as GTPase-activating proteins (GAPs).
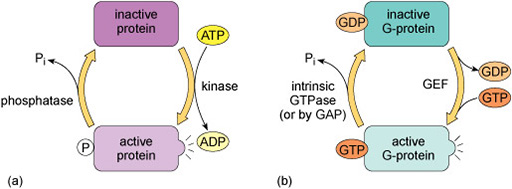
The regulation of G-proteins through binding of guanosine nucleotides is analogous to protein phosphorylation in many ways (Figure 9). In a sense, GEFs play a similar role to protein kinases in that they lead to the activation of signalling; while GAPs are comparable to protein phosphatases that terminate signalling. Moreover, just as the length of time that a protein is phosphorylated determines how long it participates in signalling processes, so the duration of G-protein signalling depends on how long the associated GTP molecule persists before it is hydrolysed to GDP.
