3.1 Problems in collieries
Before we look at any devices in detail, it is useful to examine what invention involves. Invention usually starts with a problem, and it is the solution to that problem to which the invention is addressed.
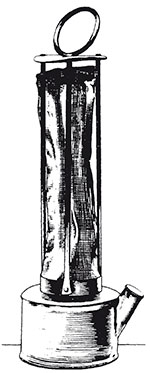
The original miners' lamp was proposed almost simultaneously by Humphrey Davy and George Stephenson to prevent explosions in coal mines. Until the invention of this lamp, mines were lit by candles. This was very dangerous because the open flame could ignite the methane gas (or 'firedamp') found in coal pits. This methane explosion could then cause a larger explosion in the clouds of coal dust disturbed by the first blast. A disaster in 1812 at Felling Colliery in County Durham killed over 50 miners, and spurred many people, including Davy, to seek a safe light source.
Davy solved this particular problem by reasoning that what was needed was a way to restrict the flame inside a shield so it could not ignite any methane outside of the lamp. However, sealing the flame completely in an enclosure would just cause it to extinguish through lack of oxygen. Davy reasoned that a metal gauze (a type of mesh) could conduct heat away and prevent a flame escaping to cause an explosion. But what size gauze to use? If the gauze mesh is too coarse, the flame will pass through the gaps. Too fine, and there will be a reduction in the light output and the shield will be too fragile. Davy showed that a maximum gap size of around 1 mm in the wire gauze was needed to prevent flame transmission, a value that he determined by experiment.
Davy never patented the invention but was rewarded by an award of money subscribed by grateful mine owners. Ironically, his important contribution (shown in Figure 38) led to a greater number of explosions in the short term. The reason was that the iron gauze could be damaged too easily and rusted very quickly. The critical mesh size was exceeded if only one interconnection was broken. Later inventors in the middle of the 19th century created better versions of the lamp by using several gauzes (the idea of fail-safe or redundancy, so that if one rusted, there were others to prevent the flame penetrating), and by using glass to surround the flame below the gauzes.
Until very recently, lamps of Davy's basic design were still in regular use for testing gaseous atmospheres. If methane gas is present, it will burn as a blue cone above the main flame (with the gauze preventing the flame from igniting the methane in the mine). The height of the cone enables the estimation of the concentration of methane in the air, from 0.5% up to several per cent.
The mine workings must be evacuated if the methane concentration reaches 1.25%, so the lamp gives critical warning of dangerous conditions. It has now been replaced by methanometers, which monitor the methane concentration using an alumina sensor soaked with palladium or platinum catalysts. However, the sensor is easily poisoned by sulphur, which is prevalent in mines, so the devices need constant and regular maintenance, and there is still much reliance on flame lamps for their ease of use and reliability.
Activity 16 (self-assessment)
- a.Describe briefly the problem which led to the invention of the flame safety lamp.
- b.What single key idea led to a solution to the problem?
- c.How was this concept translated into a working device?
- d.Indicate what practical problems arose when the device was used in collieries, and how those problems were overcome.
Answer
- a.The problem that led to the flame safety lamp was the widespread occurrence of methane explosions in collieries. They were caused by ignition of the methane by open flame lamps used for illumination in the pits.
- b.The single key idea that led to a solution of the problem was the observation that iron gauze of fine enough mesh size would prevent a methane flame penetrating through it.
- c.The idea was used by Davy to construct a safety lamp by enclosing a simple flame lamp with a cylindrical gauze capped by another piece of gauze of the same mesh size (~1 mm). Any methane gas in the colliery air would burn safely within the lamp, giving a blue cone above the bright part of the flame. It would be prevented from reaching the outer colliery atmosphere by the gauze.
- d.The main practical problem that arose as a result of use of the design in working collieries concerned rusting of the gauze. Moisture could attack and corrode the wires, and if just one such wire rusted through, the lamp became unsafe and a flame could pass through and cause an explosion. The rusting problem was solved by providing more than one enveloping gauze, so if one rusted through, another was there to provide protection. The flame could also be protected by a glass cylinder below the set of gauzes.