5.4 Batteries, chemistry and corrosion
Batteries such as the one Volta invented are devices put together to encourage chemical reactions between metals and Electrolytes. These reactions are as sure to happen as a ball is to roll downhill, though it is not so easy to understand why. Suffice to say that there is such a thing as 'chemical energy' in the same way that there is kinetic energy and gravitational potential energy. We'll get by for now with only a superficial look at the science.
The link between chemistry and electricity is not an accident. The clustering together of atoms in solids, and to a lesser extent in liquids (and barely at all in gases), is the result of the chemistry of the material in question – and this chemistry is itself affected by the electrons within the atoms. So it is both chemical and electrical.
Electrolytes
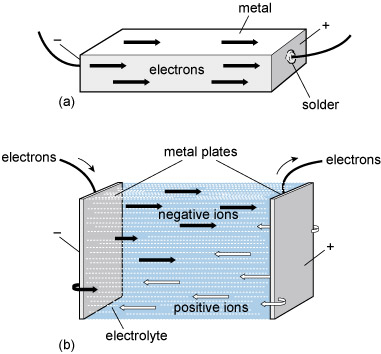
An electrolyte is a substance that conducts electricity by mechanisms distinctly different from those in metals. Normal metallic conduction is accounted for by the drifting of electrons that are free to roam around away from their parent atoms. Conduction of electricity in this way is a defining feature of metals. Electrolytes conduct electricity through a drifting of electrically charged atoms rather than electrons. Electrically charged atoms are known as 'ions'. Figure 84 contrasts conduction in metals and electrolytes.
Many electrolytes are solutions in water or some other solvent. Strong electrolytes contain a high concentration of ions and conduct electricity well. These good electrolytes include strong acids and alkalis, and most 'salts'. A salt is a chemical compound which, when dissolved, liberates positive and negative ions from their ordered positions in the solid. These released ions are then free to carry electric charges between electrodes immersed in the solution.
A battery contains an electrolyte in either a liquid or a paste-like solution. Liquid electrolytes are used in electrolysis, electroplating, and other chemical processes.
A few solids, particularly oxides, conduct electricity ionically (meaning through the movement of charged atoms) at temperatures close to their melting point. A few other substances exhibit ionic conduction when they are in a molten state.
Whenever chemical rearrangements – or 'reactions' – occur, atoms are required to swap or share electrons with each other. Energy is bound up in any arrangement of atoms. Where the result of a rearrangement binds up less chemical energy than before, chemical potential energy is released. In some cases the energy is released as electrical energy, just as a rolling ball can transfer its potential energy into kinetic energy as it rolls down a hill. The cell within a battery is configured to intercept these transactions so that the electrical energy made available by the chemical reorganisation can be gathered up. Ultimately things are arranged so there is a separating of electrical charge, driven by chemistry. You may already know that we call this subsequent pushing and pulling of charges an electromotive force or emf, which is denoted and measured in volts.
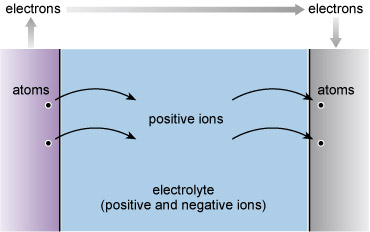
In the case of batteries, the chemical rearrangements involve relocating atoms from the electrolyte onto the surface of one of the 'two dissimilar metals' that Volta prescribed. At the surface of the other metal, atoms pass into the electrolyte (Figure 85). The electricity is inseparable from the chemistry here as the atoms leaving the electrolyte are electrically charged (positive) and more properly called positive ions. These atoms collect negative charges from the metal as they pass from the electrolyte onto the metal surface, so becoming neutral atoms once more. Similarly atoms of the dissolving electrode leave negative charge behind and enter the electrolyte as positive ions.
A battery will be spent when the electrode that is giving up ions to the electrolyte has been completely consumed, or rather 'dissolved'. Metals that are consumed in this way are said to be undergoing corrosion. In a similar way, I am certain that the bottom of my wheelbarrow is spent when rust has eaten a hole through which I can see the ground. Chemically the processes in a battery and in the Corrosion of steel have a great deal in common: two dissimilar metals and an electrolyte are all in contact.
Chemical reactions that occur spontaneously, such as corrosion, are useful for generating energy. Sometimes, by careful design, we can divert the energy into electricity, otherwise it usually ends up as heat, light, or sound. In some cases, we can even use electricity to drive the chemistry backwards. This is the secret of rechargeable batteries, which we will come to in the next section. It's also behind a clever strategy for inhibiting corrosion called Galvanic protection.
Corrosion of steel
Steel is not the only metal that corrodes in the atmosphere, but as a major structural metal it's the one of which we are most aware. The iron in steel was extracted from an ore in which it was in a stable chemical combination with oxygen (and to a much lesser extent with other elements). As it corrodes it is simply returning to that stable state as inexorably as a ball rolls downhill.
Corrosion occurs whenever steel is in contact with air and moisture, which acts as an oxygen-bearing electrolyte. The 'dissimilar metals' requirement we mentioned earlier can be provided initially by microscopic variations in the steel composition, or in the oxygen concentration of the moisture with which it is in contact.
In the corrosion process, chemical rearrangements occur because, chemically speaking, a 'more efficient' arrangement can be found that lowers chemical potential energy.
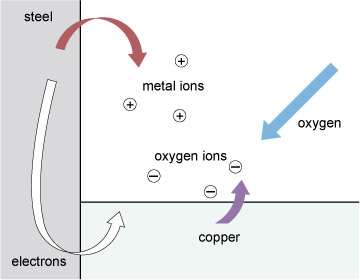
Figure 86 shows the process schematically, with the steel joined to a dissimilar metal to make things easier to follow. Of the two dissimilar metals, one, and always the same one for any given pair, will be the preferred local host for any oxygen and this is the one that corrodes. Atoms of this metal swap their own electrons for other negative charge (electrons) stuck to atoms of oxygen. This process, the familiar hallmark of corrosion, is marked by the red arrow in Figure 86. The metal atoms leave the metal and enter the electrolyte 'in search of oxygen'.
The other metal cooperates in two ways. First it takes in refugee electrons from the dissolving material; a local electric current is always associated with corrosion. Second, it takes part in the hand over of electrons to oxygen atoms, indicated by the mauve arrow. The final products of corrosion build up in the electrolyte.
Corrosion of steel is one of the most familiar examples of oxidation, rust being a mixture of iron oxides. Oxygen has quite a reputation as an aggressive collector of two electrons per atom; in fact among chemists it's common practice to refer to 'oxidation' when atoms surrender electrons to other atoms even if oxygen isn't directly involved.
You are likely to have seen corrosion if you've looked at ships in harbours. Scratches in the paintwork expose the steel. The 'dissimilar metals' in this case extends to the difference between painted and bare steel. Corrosion proceeds under the paint, causing it to blister and peel off, exposing more steel (see Figure 87).
Galvanic protection
When dissimilar metals are in contact in the presence of an electrolyte, corrosion may occur. Knowing this, could we use it to our advantage? We would need to know, in the first instance, which of the two metals corrodes. The answer is in Volta's ordering of bad-tasting metals that was presented in Section 4.2. Following additional experiments, Volta revised the order of his list:
- zinc, lead, tin, iron, copper, platinum, silver, gold, graphite.
Taking this further, Table 5 lists the galvanic series. It orders metals in terms of corrosion, and is determined by slightly more sophisticated methods than Volta's 'suck it and see'!
When two of these metals are connected together in the presence of an electrolyte, the uppermost in the list will be the one which corrodes.
Notice the similarities between Volta's list and the galvanic series. In Volta's list there are some differences in the ordering but this is probably related to the purity of the material available to him.
| Chemical symbol | Element name |
|---|---|
| Mg | Magnesium |
| Al | Aluminium |
| Ti | Titanium |
| Zn | Zinc |
| Fe | Iron |
| Cd | Cadmium |
| Ni | Nickel |
| Sn | Tin |
| Pb | Lead |
| Cu | Copper |
| Ag | Silver |
| Au | Gold |
From the order given by the galvanic series one can pick out two familiar combinations:
- Galvanised steel : zinc coatings on steel afford an excellent protection against rusting; where the coating is scratched and steel (iron) and zinc are joined in the presence of water (the electrolyte) it is the zinc that corrodes.
- Tin-plated steel : tin plating will prevent rusting of steel as long as the coating remains entire; if scratched or cracked then corrosion of the exposed steel (iron) will be driven by the tin whenever an electrolyte (e.g. water) is present. Dented or scratched tin cans are not the best packaging for food!
Here are a couple of less well-known combinations:
- 'Sacrificial metals' : zinc (or aluminium) blocks bolted to the steel hulls of large ships inhibit corrosion as the zinc (or aluminium) block preferentially corrodes.
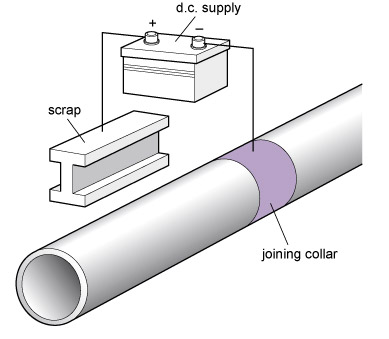
- Impressed current : because a current flows between the corroding metals, a battery or other source of e.m.f. can be used to push charges the other way, so inhibiting corrosion; see Figure 88.
Historically, anti-corrosion measures based on the galvanic series have been referred to as 'sacrificial anodes' and 'cathodic protection'. For our purposes, I prefer to reduce the risk of confusion with some of our electrical terminology by using the more general expression 'galvanic protection'.