We all know that air is essential for human life; more precisely, the oxygen in air is essential for life. A human breathes in approximately 11 000 litres of air every day. But how is that oxygen transported into and around our blood systems and stored in the parts of our body that need it to function? And are humans different to other organisms in how we use oxygen?
 Jump to:
Jump to:
- The oxygen in our blood
- Haemoglobin & myoglobin
- Why can blood be different colours?
- Green blood? Science fiction or science fact?
Overview
In this article you will discover that:
- many organisms need a protein to transport oxygen through the blood
- these proteins are metalloproteins - they contain a transition metal ion
- the transition metal ion must be able to be oxidised and reduced as it binds and releases oxygen
- these different oxidation states lead to different colours and
- different organisms use different metal ions and so their blood displays different colours.
The chemistry of the oxygen in our blood
Oxygen (O2) is transported via the bloodstream from the lungs to all parts of our bodies. The oxygen diffuses from the bloodstream into the cells, where it is used in aerobic respiration, the major process that provides energy. Using glucose as an energy source, the overall reaction for aerobic respiration is:
C6H12O6(s) + 6O2(g) = 6CO2(g) + 6H2O(l) + energy
Six moles of oxygen are consumed for every mole of glucose, and a good supply of O2 is essential to enable our cells, and bodies, to function normally. Similarly most organisms, from the smallest single-cell amoeba to the largest elephant depend on supplies of O2 to survive.
Single cell organisms
For small, single-cell organisms, oxygen is easily obtained. These organisms utilise the slightly soluble of oxygen in water and its ability as a small molecule to be able to quickly penetrate or diffuse through cell membranes.
What is passive diffusion of O2?
O2 transfers across a biological membrane, involving no expenditure of energy, moving from an area of high concentration to a lower one.As single cells are very small (ranging from 1 to 100 μm in diameter), the passive diffusion of oxygen into the centre of the cell is fast enough to support the respiration reactions.
However, the amount of oxygen that can diffuse passively through the cell drops off rapidly with the distance over which the oxygen has diffused. Consequently organisms that rely on the passive diffusion of oxygen cannot be larger than about 1 mm in diameter; for larger organisms the oxygen would not get through in large enough quantities to support respiration.
Temperature is also important. The solubility of oxygen in water falls with increasing temperature. At 5 °C the solubility of oxygen in water is about 2 mmol dm−3, which is enough oxygen in solution to maintain the respiration rate of a unicellular organism. Thus, very small organisms living at temperatures of about 5 °C are able to obtain their oxygen requirement by passive diffusion. However, at 40 °C the solubility falls to around 1 mmol dm−3.
But what about larger organisms, ie humans?
Two problems must be overcome to supply sufficient oxygen to large organisms (like humans), namely:
- The rate of passive diffusion of oxygen through respiring tissue (e.g. skin) is not fast enough to penetrate much further than about 1 mm.
- The solubility of oxygen drops off with increasing temperature.
The solubility of oxygen in blood plasma (the fluid component of blood, which does not contain red blood cells) at 37 °C is 0.3 mmol dm−3. So, for warm-blooded organisms, like humans, the solubility of oxygen in blood plasma is not high enough to support aerobic respiration in the cells.
Why does the ice-fish have no biochemical oxygen concentration system?
At these temperatures the solubility of oxygen in water (or colourless blood) is higher even than at 5 °C, high enough to support respiration in the cells of the fish, so it has no need of a chemical system to concentrate oxygen in its bloodstream. The solubility of oxygen in water at −1 °C is about 5 mmol dm−3.To survive, large animals (that is, greater than 1 mm in size) must have a means of capturing oxygen from the air, circulating it around their body and, if they are warm-blooded or exist in hot climates, find a way of concentrating oxygen within their circulation systems.
The first problem of circulation is largely a mechanical one; requiring a pump and pipes namely the heart and blood vessels. The second problem of increasing the concentration of oxygen within circulation systems is largely a chemical one. It is this problem and the biochemical systems that overcome it, which will be the focus of this section.
As a final thought, consider the Antarctic ice-fish. This fish has a heart and circulation system similar to all vertebrates. However, it has no means of concentrating oxygen in its bloodstream (in fact, its blood is completely colourless). These fish live in temperatures of about −1 °C.
Haemoglobin and myoglobin
From the introductory discussion it is apparent, larger organisms must have a system for concentrating and circulating O2 within their bodies; otherwise the passive diffusion of O2 into the interior of the organism would be too slow to support aerobic respiration reactions. From a chemical point of view, it is seen that such organisms will use the chemical properties of transition metals in O2 transport systems. We shall also see that another property of transition metals – the ability to form highly coloured complexes – is useful in characterising any transition metal-containing protein we study.
The brilliant red colour of blood comes directly from a chemical group called haem, which contains the transition metal iron. More specifically, the haem is found in the blood’s O2-carrying protein, haemoglobin (Hb) and storage protein, myoglobin (Mb). Haemoglobin is present in the bloodstream of many organisms.
Myoglobin
Myoglobin (Mb) is found exclusively in muscle tissue, where it acts as an oxygen storage site and also facilitates the transport of oxygen through muscle. As muscles require a lot of energy to operate, they also require very efficient access to oxygen in order to maximise respiration rates.
Both Hb and Mb have been the subject of intensive study. But what we know about both proteins comes almost entirely from a knowledge of their three-dimensional structures. The structures of myoglobin and haemoglobin were determined by J. C. Kendrew and M. F. Perutz, respectively.
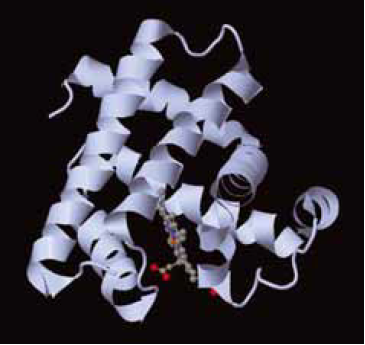 The higher-order structure of myoglobin, showing the large alpha-helical content of the protein. The haem is shown in so-called a ball and stick representation.
The higher-order structure of myoglobin, showing the large alpha-helical content of the protein. The haem is shown in so-called a ball and stick representation.
The protein consists mostly of α-helices of polypeptide. Sandwiched between two long α-helices lies a porphyrin ring. The porphyrin in Mb has various side chains attached to the outside of the ring. This particular derivative is known as protoporphyrin IX.
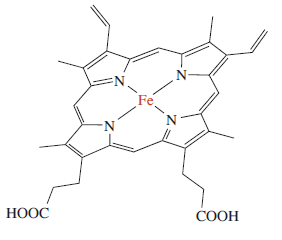 The chemical structure of an iron porphyrin.
The chemical structure of an iron porphyrin.
Here an iron(II) atom lies at the centre of the ring, coordinated by the four nitrogen atoms of the pyrrole groups. Note that the whole haem group (apart from the side chains) is planar. The double bonds shown above are for one resonance structure; in reality the porphyrin is extensively delocalised. Mb, like Hb, has a strong red colour, which arises from π–π* transitions within the extensively delocalised haem group. On oxygenation of deoxy-Mb the colour of the protein changes from a dull red to a bright red as shown in the visible absorption spectrum below. This colour change identifies the haem group as the site of O2-binding within the protein.
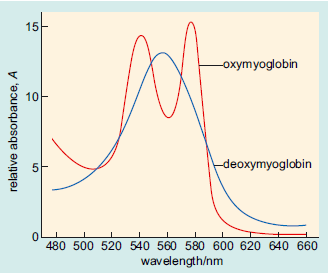 Visible spectra of oxymyoglobin and deoxymyoglobin. The bands are due to pi-pi* transitions within the haem group. The fact that the spectrum changes on oxygenation shows that the haem group is the oxygen-binding site within the protein.
Visible spectra of oxymyoglobin and deoxymyoglobin. The bands are due to pi-pi* transitions within the haem group. The fact that the spectrum changes on oxygenation shows that the haem group is the oxygen-binding site within the protein.
Haemoglobin
Haemoglobin (Hb) occurs in the red blood cells (erythrocytes) of many organisms, where it concentrates O2 in the bloodstream and transports it around the organism. Red blood cells are packed with Hb, which usually constitutes about 33% by mass of the cell. Whole blood contains about 120–180 g of Hb per dm3. The solubility of O2 in blood at 37 °C is about 10 mmol dm−3 compared to the solubility of O2 at 37 °C in water, which is about 1 mmol dm−3 and in blood plasma, which is about 0.3 mmol dm−3.
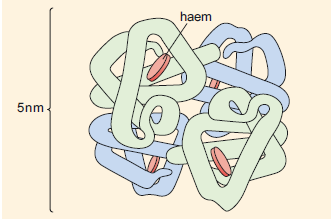 The quaternary structure of haemoglobin. The haem groups (shown in red) are non-protein planar structures with an iron atom at the centre where the oxygen is bound. The four polypeptide chains are shown in blue or green.
The quaternary structure of haemoglobin. The haem groups (shown in red) are non-protein planar structures with an iron atom at the centre where the oxygen is bound. The four polypeptide chains are shown in blue or green.
At first sight the structure of Hb appears to be very complex. However, it is easier to consider Hb as essentially four Mb units linked together. Therefore, the Hb molecule has four haem units and, hence, four O2-binding sites. The active sites of the haem units in Hb are almost identical to the active site structure in Mb, complete with proximal and distal histidines.
Why do some animals have different coloured blood?
Some animals have different coloured blood from the brilliant red colour of blood that contains Hb. These different colours are qualitative evidence that the O2-carrying protein in these animals is not Hb:
- some invertebrates have blood that is colourless in the absence of O2 yet a deep blue colour in the presence of O2 (true blue blood); the blood of certain marine worms is colourless in the absence of O2 and deep burgundy in the presence of O2.
- that all these species have coloured blood suggests that their O2 transport systems could involve transition metal-containing proteins. This arises because many transition metals other than iron can exist in a variety of oxidation states, and this is the key property for metals in O2-carrying proteins.
Haemerythrin
Haemerythrin (Hr) occurs in the bloodstream of certain marine worms. Its higher-order structure has similarities to Hb (which we can consider to be a Mb tetramer), in that Hr is often found as an octameric unit, with a total relative molecular mass of about 8 × 13 500 = 108 000. At its active site, Hr contains two iron atoms. However, despite the ‘haem’ in its name, the protein does not contain a haem group.
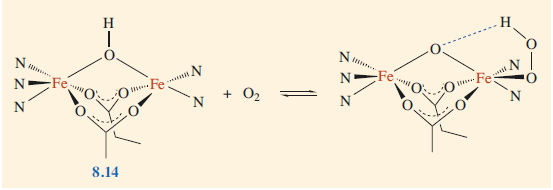 Binding of oxygen to haemerythrin and structure of the active sites. (where N and O groups bound to the iron originate from amino acid side chains within the protein).
Binding of oxygen to haemerythrin and structure of the active sites. (where N and O groups bound to the iron originate from amino acid side chains within the protein).
Haemocyanin
The last type of O2-carrying protein we shall study here is called haemocyanin (Hc). This protein occurs in the bloodstreams of molluscs (e.g. snails) and arthropods (e.g. spiders). It is a very large protein with a relative molecular mass of around 1 000 000. In the deoxygenated state it is colourless, whereas in the presence of O2 it is blue. At its active site, Hc has two copper atoms. In fact, the name ‘haemocyanin’ is a double misnomer, as the protein contains neither a porphyrin ring nor an iron atom, although it is blue as reflected in ‘cyan’.
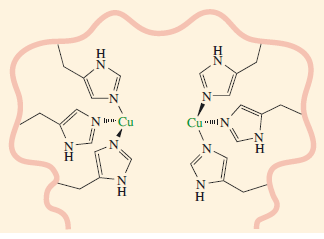 Active site of deoxyhaemocyanin. The copper-to-copper distance is about 360 pm (for scale, each copper-to-nitrogen bond distance is around 190 pm, Haze et al. 1993).
Active site of deoxyhaemocyanin. The copper-to-copper distance is about 360 pm (for scale, each copper-to-nitrogen bond distance is around 190 pm, Haze et al. 1993).
 Green blood in humans? Science fiction or science fact?
Green blood in humans? Science fiction or science fact?
Green blood featured in an episode of the crime drama CSI: Crime Scene Investigation (The Theory of Everything). It may sound like science fiction - and indeed Star Trek’s Mr Spock supposedly had green blood - but real cases have been noted of patients whose blood is a dark green.
The cause of the colour is the replacement of haemoglobin by sulfhaemoglobin. In sulfhaemoglobin, there is substitution of sulfur into the porphyrin ring. Spectroscopic evidence has been recorded that suggests that the porphyrin group has been converted into a chlorin ring containing sulfur. The uptake of O2 is reduced in patients whose blood contains sulfhaemoglobin as it binds O2 less strongly. Sulfur can be incorporated into haemoglobin from sulfur-containing drugs. In a case in Canada (Flexman et al., 2007), as in the CSI episode, the patient had been taking large doses of a sulfur-containing migraine drug.
These topics along with many others are covered in the Open University level 3 course S315 Chemistry: further concepts and applications where this article was adapted from.
Explore articles and free courses or study with the OU
References
Flexman, A.M., Del Vicario, G. and Schwarz, S.K.W. (2007) ‘Dark Green Blood in the Operating Theatre’, The Lancet, vol. 369, p. 1972.
Hazes B., Kalk K. H., Hol W. G. J., Magnus K. A., Bonaventura C., Bonaventura J. and Daute Z., (1993) ‘Crystal structure of deoxygenated Limulus polyphemus subunit II hemocyanin at 2.18 A resolution: clues for a mechanism for allosteric regulation’, Protein Science, Vol. 2, pp. 597-619.
Hersleth, H.P., Uchida, T., Røhr, A.K., Teschner, T., Schünemann, V., Kitagawa, T., Trautwein, A.X., Görbitz, C.H. and Andersson, K.K. (2007) ‘Crystallographic and spectroscopic studies of peroxide-derived myoglobin compound II and occurrence of protonated FeIV O’, Journal of Biological Chemistry, vol. 282, no. 32, pp. 23372–86.

Rate and Review
Rate this activity
Review this activity
Log into OpenLearn to leave reviews and join in the conversation.
Activity reviews