2.1.2 Ammonia and synthetic nitrogen fixation
Ammonia is a colourless gas with a very strong characteristic smell. It was first produced industrially by the Haber-Bosch process in Germany (Equation 8).
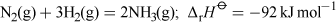
At room temperature the reaction is very slow, which means industrial processes must operate at high temperature (400-450 °C) and high pressure (8-35 MPa) and also require the presence of an iron catalyst. The fertilisers ammonium nitrate, NH4NO3, and ammonium sulfate, (NH4)2SO4, are manufactured by treating ammonia with either nitric or sulfuric acid, respectively.
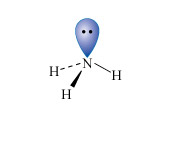
Nitrogen is the first element of the second row of the Periodic Table to have a non-bonded pair of electrons in its normal valency state; this is typified by the ammonia molecule (Structure 2). Consequently, ammonia is a reasonably strong Lewis base (electron donor), particularly towards transition metal ions.
Nitrogen is also among the most electronegative elements and this affects both the physical properties and reactivities of its compounds.
Consider the boiling temperatures of the hydrides on descending Group 15: NH3, −33.4 °C; PH3, −87.7 °C; AsH3, −62.4 °C; SbH3, −18.4 °C. Why does ammonia have an uncharacteristically high value?
Ammonia has a higher than expected value due to the extra intermolecular forces or hydrogen bonds between its molecules.
Watch Video 1 and then answer the question following it.
Transcript: Video 1 The reaction of gaseous ammonia with hydrochloric acid.
After watching Video 1, suggest an equation for the reaction of ammonia with hydrochloric acid.
The white 'smoke' comprises small particles of ammonium chloride (Video 1):
NH3(g) + HCl(g) = NH4Cl(s)(Equation 9)
Ammonia forms an alkaline solution in water, often used in household cleaners.
Liquid ammonia is additionally a widely used non-aqueous solvent. Now watch Video 2 and answer the question following it.
Transcript: Video 2 The dissolution of sodium in ammonia.
What is seen upon the dissolution of sodium in ammonia in Video 2?
Alkali metals, such as sodium, dissolve reversibly to give blue solutions (in contrast to their more familiar vigorous reactions with water). The blue colour is thought to arise from electrons solvated by ammonia molecules.
