2.1.3 Ammonium nitrate fertiliser
Nitric acid has many uses, but most is combined with ammonia to produce the important fertiliser ammonium nitrate. Nitric acid, HNO3, is made on a huge industrial scale by the Ostwald process: involving ammonia oxidation in two stages over a platinum metal catalyst, first to NO and then to NO2 (see Video 3):
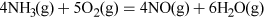
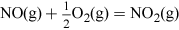
Transcript: Video 3 The oxidation of ammonia.
In Video 3 why did the platinum catalyst continue to glow?
The exothermic reaction in Equation 10 heated the platinum.
The NO2 is then dissolved in water to give a concentrated aqueous solution of the acid, and the NO produced in this step is recycled back into earlier stages. The overall reaction can be summarised by the following equation:
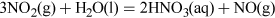
The anhydrous acid can be produced by distillation, and is a colourless pungent liquid.
Structure 3 shows the planar nitric acid molecule:
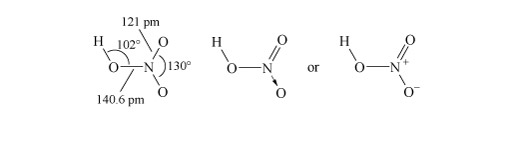
In dilute aqueous solution, nitric acid behaves as a typical strong acid, dissociating completely into H+ and NO3− ions.
Predict using valence-shell electron-pair repulsion, VSEPR theory (valence-shell electron-pair repulsion theory) t the shape of nitrate, NO3−.
The Lewis structure and structural formula of one resonance form of NO3− are in Structures 4 and 5. This adopts a planar shape because there are only three repulsion axes and no non-bonding pairs:
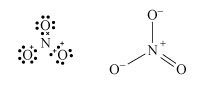 Structures 4 (left) and 5 (right)
Structures 4 (left) and 5 (right)
Ammonium nitrate fertiliser must be packed and handled with extreme care; in addition to being thermally unstable at high temperatures, its decomposition is catalysed by many inorganic and organic materials.
Strict regulations governing the storage and transportation of the chemical were introduced following explosions in 1947 aboard two American ships transporting fertiliser-grade ammonium nitrate to Europe. At the time of writing, in 2013, this remains the worst industrial accident in US history. In 2013 at Waco in Texas, a large explosion occurred at a fertiliser plant that stored ammonium nitrate.
Today, ammonium nitrate is used extensively in industrial explosives and propellants. For instance, commercial blasting explosive contains ammonium nitrate, fuel oil and TNT.
Video 4 shows that upon gentle heating ammonium nitrate decomposes.
Transcript: Video 4 Heating nitrates.
Taking it a stage further, we can demonstrate the oxidising power of potassium nitrate by melting some, and adding a piece of charcoal. In this vigorous reaction, the gases evolved are carbon monoxide, carbon dioxide, and nitrogen. This is not too far removed from the chemistry of gunpowder, where charcoal and sulfur act as fuels, which are oxidised highly exothermically by potassium nitrate.
Suggest an equation for the gentle heating of ammonium nitrate.
- NH4NO3(s) = N2O(g) + 2H2O(l)(Equation 14)
However, upon heating above about 250 °C, an alternative explosive decomposition occurs:
Potassium nitrate, known as saltpetre, is a component of gun powder (Video 4). The explosive material consists of sulfur and charcoal (a fuel), mixed with potassium nitrate (an oxidiser).
A simplified equation for the deflagration (a self-propagating burning surface reaction) of gunpowder is:
In Videos 3 and 4 you have seen examples of the production of some of the many oxides of nitrogen, namely nitrogen monoxide, NO, nitrogen dioxide, NO2, and dinitrogen monoxide, N2O. It is noteworthy that in biology NO adopts several key roles such as in the body where NO plays an important role in the regulation of blood pressure.
Nitrates of almost all of the metallic elements are known. They are all soluble in water which means that they are highly mobile in the environment. Consequently the EU regulates the application of nitrogen containing fertilisers through the EU Nitrates Directive.
