3.3 Condensation of oxoacids
The tendency for an oxoacid to polymerise by condensation is most marked in the less acidic (more highly hydroxylated) acids. Consequently there are many stable condensed forms of silicic and boric acid which are found in minerals.
Condensation is most marked in structures where the charge on the uncondensed anion is high, because it is able to reduce the charge density on the anion. For example SiO44− dimerises as follows:
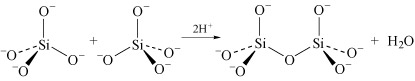
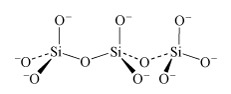
In the monomer there are four negative charges for four oxygen atoms; in the dimer there are six negative charges and seven oxygen atoms; in the trimer (Structure 8) there are eight negative charges and ten oxygen atoms.
In the limiting case of the infinite polymer, there are two negative charges to every three oxygen atoms.
What is the repeat unit in Structure 8 upon double protonation of the individual unit?
In the case of protonation by two H+ per Si, the repeat unit is [H2SiO3].
This is the structural unit found in the pyroxene minerals (Figure 11). Here shared SiO4 tetrahedra can be assembled into chains, double chains, sheets, rings and three-dimensional networks to give an amazing variety of structures for crystalline silicate minerals.

