4.1 Aqueous chemistry of aluminium
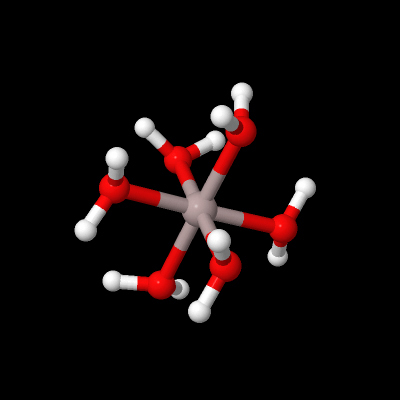
Aluminium is a metal forming an aqueous cation, unlike the non-metal boron found above it in Group 13. For example, hydrates of aluminium sulfate, Al2(SO4)3, can be made by dissolving bauxite, Al2O3.3H2O, in sulfuric acid and evaporating the solution. Dissolving such sulfates in water forms Al3+(aq) (Figure 16):
What is the coordination geometry of aluminium in Figure 16?
Al3+(aq) is shorthand for the octahedral coordination complex Al(H2O)63+.
Careful addition of aqueous sodium hydroxide to this solution will first precipitate insoluble aluminium hydroxide:
The oxide is produced by filtering and heating the hydroxide:
Both oxide and hydroxide are unusual in being amphoteric. Hence they will first dissolve in and neutralise acids:
Watch Video 5 to determine how else an amphoteric hydroxide (or oxide) behaves.
Download this video clip.Video player: Video 5Transcript: Video 5 The reaction of aluminium(III) ions with hydroxide ions.
End transcript: Video 5 The reaction of aluminium(III) ions with hydroxide ions.NARRATORThis is an aqueous solution of potassium aluminium sulfate, often referred to as 'alum'. It contains the aluminium three plus ion. If we add dilute sodium hydroxide, we precipitate aluminium hydroxide, an insoluble base used in indigestion tablets. However, in excess alkali, we form the soluble aluminate ion.Video 5 The reaction of aluminium(III) ions with hydroxide ions.Interactive feature not available in single page view (see it in standard view).It will dissolve in an alkali and will neutralise it.
As excess sodium hydroxide is added to the precipitate that is initially formed in Equation 37, the precipitate dissolves to form the tetrahydroxyaluminate ion:
This shows a close resemblance of Al(OH)3 to Be(OH)2, which is also amphoteric. Note that the bases ammonia and sodium carbonate are not strong enough to cause this dissolution.