4.1.3 Aluminium toxicity
The exact mechanism of aluminium toxicity remains controversial. Aluminium has been shown to disrupt lipid membrane fluidity, perturb iron, magnesium, and calcium regulation or so-called homeostasis, and cause oxidative stress by upsetting the normal treatment of unwanted oxidising radicals by the body.
Why is the observation that aluminium causes oxidative stress perhaps surprising?
Alumunium, unlike transition metals, does not have redox chemistry as it only forms the +3 ion. Therefore it is thought that aluminium(III) ions interfere with enzymes which counter reactive oxygen species (ROS) in the body.
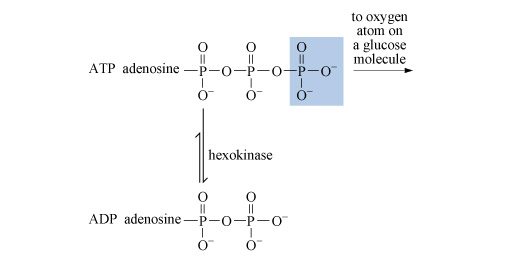
Glycolysis is a nearly universal pathway in biological systems which aluminium can influence. Glycolysis includes a key step in which adenosine triphosphate (ATP) loses its terminal phosphate group to a glucose molecule and becomes adenosine diphosphate (ADP) (Figure 19).
The process is catalysed by an enzyme called hexokinase. Before the ATP can be accepted by the enzyme, it must become complexed to a magnesium ion, Mg2+, through oxygen atoms attached to phosphorus atoms 2 and 3 (Structure 12). The exact way in which the magnesium ion bridges the two terminal phosphates is uncertain, but in this complexed state, the ATP is accepted by the enzyme. The magnesium ion then moves from the bridging position between phosphate groups 2 and 3 to one between groups 1 and 2. This exposes the terminal phosphate group to removal, and transfer to glucose.
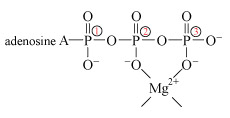
The change of bridging position is possible because Mg2+ does not bind particularly strongly to the oxygen donor sites. Now suppose substantial amounts of dissolved aluminium are present:
What ion is then available for binding to the oxygen donors?
Al3+(aq), which from simple electrostatic arguments will bind more strongly than Mg2+ to oxygen donor ligands.
Consequently Al3+ will bridge phosphate groups 2 and 3 like Mg2+, but because of the stronger binding, it is reluctant to shift to bridge groups 1 and 2 upon binding by the enzyme. Loss of the terminal phosphate is therefore impaired.
Whether this explanation of aluminium neurotoxicity is the whole story or not, it still points to a useful general idea about poisons. Their key feature is a resemblance to some chemical species (in this case Mg2+) that is essential to a biological process, and which is similar enough to gain access to a metabolic process. The poison once incorporated produces differences from the essential species and the metabolic process is blocked.
In natural waters the concentration of aluminium is increased by acid rain. When the hydrogen ions of acid rain fall upon soils that are naturally quite acidic, they replace other positive ions bound into the soil structure, such as Al3+.
For instance, the common mineral feldspar occurs widely in many different rock types, and is broken down during weathering by both physical and chemical degradation processes. Feldspar typically weathers as follows:
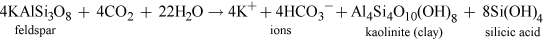
Kaolinite can react with water to give aluminium hydroxide (gibbsite) and hydrated silicic acid. The aluminium hydroxide dissolves in the presence of hydrogen ions.
Suggest an equation for the dissolution of aluminium hydroxide in acid.
From earlier:
Al(OH)3(s) + 3H+(aq) = Al3+(aq) + 3H2O(l)(Equation 48)
This aluminium contamination is a major contributor to the decline of fish stocks in lakes. The Al3+ ion appears to bind to oxygen-donor ligands, such as the phosphate groups in phospholipids, at the surface of fish gills. This makes the membrane of gill cells more permeable to further Al3+, which can then enter the cells and replace Ca2+ ions on key proteins. The regulation of the cell concentrations of other ions, such as Na+, is also disrupted. At the same time, the increased concentration of aluminium in gill cells leads to precipitation of aluminium hydroxide, mucus formation and breathing difficulties.