3.4 Benzodiazepine tranquillisers, Prozac and the SSRIs
One of the most significant ranges of drugs ever produced is the benzodiazepine tranquillisers (usually classed as ‘minor tranquillisers’ or ‘hypnotics’), often prescribed as a remedy for ‘minor’ disorders such as depression, sleeplessness and anxiety. In effect, they extended the range of conditions that could be treated by medication. The best-known example is probably Valium.


Like their predecessors, these drugs began to attract criticism from a number of different quarters. Some pointed to the dangers of addiction (Agrawal, 1978), while others said that the drugs were being used to help women endure gender oppression rather than escape it (Johnstone, 1989; Waldron, 1977). The selected adverts reproduced here certainly suggest a focus on ‘women’s problems’.
Addiction to benzodiazepines continues to be a major topic of controversy. In 2001 a study conducted for the BBC TV programme Panorama (The Tranquilliser Trap, broadcast 13 May 2001) showed that 28 per cent of people being prescribed benzodiazepines had been taking them not for the recommended four weeks, but for more than ten years. Although more recent debates have centred around the importance of legitimate use of benzodiazepines, where they should be appropriately used alongside psychotherapy (i.e. talked-based interventions) (Silberman et al., 2020).
In 1987 came Prozac (fluoxetine), one of the range of drugs known as the selective seratonin re-uptake inhibitors (SSRIs). According to trials commissioned by the drug companies, these new drugs were non-addictive and had fewer side effects. They were marketed alongside benzodiazepines for a wide range of conditions from ‘generalised anxiety disorder’ to severe depression and schizophrenia. These drugs aim to actually ‘cure’ the ‘illness’, arguably taking mental distress further out of its social and holistic context. In the USA, SSRIs have been marketed directly to the public in an effort to get people to persuade their doctors to prescribe them. SmithKline Beecham spent $30m marketing the SSRI Seroxat (Paxil) directly to customers in the USA in 1999 alone (Rogers and Pilgrim, 2003). Marketing campaigns began to hint that as well as curing depression and anxiety, SSRIs could enhance functioning in healthy people.
Such direct marketing is banned in many countries including the UK, but the media were nevertheless susceptible to stories planted about the new ‘wonder drugs’. The government responded to the problem of keeping health-related costs under control by creating in 1999 the National Institute for Health and Care Excellence (NICE) (previously called the National Institute for Clinical Excellence), a semi-autonomous body charged with deciding which interventions are evidence-based and should be used in practice.
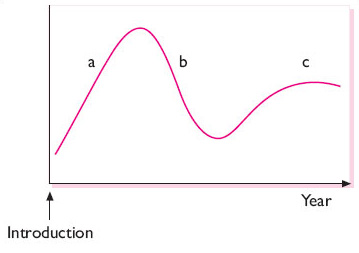
Beginning in the 1990s evidence emerged that SSRIs might not be as safe as originally thought (Breggin, 1993). The cycle of acceptance for new drugs (and health interventions more generally) such as the SSRIs, as with their predecessors, has followed the pattern shown below in Figure 6. The graph shows a peak reflecting initial enthusiasm that there will be a cure, followed by devaluation as side effects and issues become more evident, followed by stability as ‘rational use’ is established.
In summary, then, although medication has had a beneficial effect on many millions of people experiencing mental distress, there remain serious concerns in particular the influence of pharmaceutical companies on medical personnel, the research agenda, university departments and potentially even governments.