3.1 The electromagnetic spectrum
Electromagnetic radiation is defined as energy in the form of waves that have both electrical and magnetic properties. The electromagnetic spectrum covers a continuous range of wavelengths. The energy of electromagnetic radiation depends on the wavelength of the radiation. The shorter the wavelength, the higher the energy associated with that radiation.
Figure 5 illustrates the range of the electromagnetic spectrum and the uses to which the radiation is put. At the highest energy (shortest wavelength) are gamma rays which are produced during some nuclear radioactive decay processes (including nuclear fission in nuclear weapons). Gamma rays are highly destructive to life and penetrate most matter. At the lowest energy (longest wavelength) in the spectrum are radio waves which are used to transmit radio and television signals across large distances.
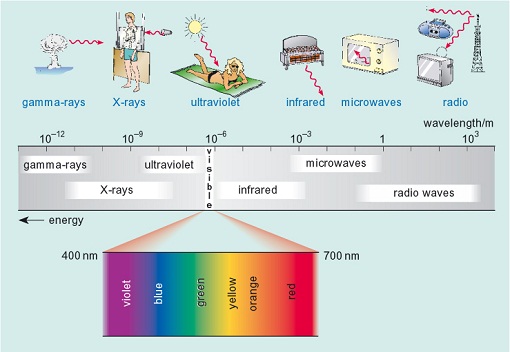
In Figure 5, the value of the wavelength of the electromagnetic radiation is between about 10-12 m and 103 m (i.e. between 0.000 000 000 001 and 1000 metres). Note that the highest energy radiation has the shortest wavelength. Gamma rays (10-12 m) are very high energy while radio waves (1-103 m) are very low energy.
Question 7
Using the information in Figure 5 arrange the following types of radiation in order of increasing energy : infrared; ultraviolet; microwave; visible.
Answer
Figure 5 shows that the wavelength of electromagnetic radiation decreases from radio waves, on the right of the figure to gamma rays, on the left of the spectrum. The shortest wavelength radiation has the highest energy. Therefore the energy increases from the lowest energy of the four in the question, microwave, to infrared and then to visible and finally to the highest energy of these, ultraviolet radiation.
