2.1.3 Candidate genes and dopamine
Recall from Session 1 that several candidate genes have been identified as risk factors for ADHD and that these often relate to dopamine. To understand how these might confer risk for ADHD, you need to understand more about the process of neurotransmitter use within the brain (Figure 9).
Neurons send messages to one another by releasing neurotransmitters across gaps between them. These gaps are known as synapses (or synaptic clefts). The signal for a neuron to release neurotransmitter into a synapse is electrical, in the form of an action potential. As shown in Figure 9, the action potential travels along the neuron until it reaches the section close to the synapse where neurotransmitter is stored. Once the neurotransmitter has been released, it can only pass on the communication if it is able to join to receptors located on the neighbouring neuron. You can think of this as being like a key fitting into a lock, where the neurotransmitter is the key, and the receptor is the lock. Once neurotransmitter has crossed the synapse and slotted into the receptors it is released from them to float back into the synaptic cleft (the gap between two neurons).
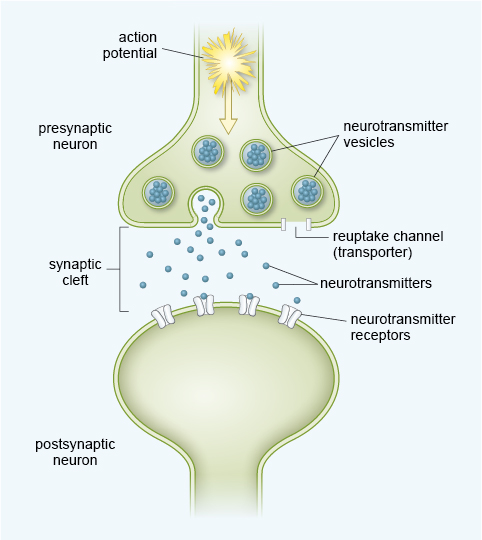
Released neurotransmitter must be removed from the synapse in some way, otherwise the communication signal would not have a discrete end point.
It is known that neurotransmitters can be removed by the process of reuptake, through special reuptake channels (also called transporters) which take the neurotransmitter back into the presynaptic neuron, so it can be released again in future. Neurotransmitter can also be removed by enzymes which break it down. Finally, neurotransmitter can also eventually disperse away from the synapse so that it is no longer near the receptors.
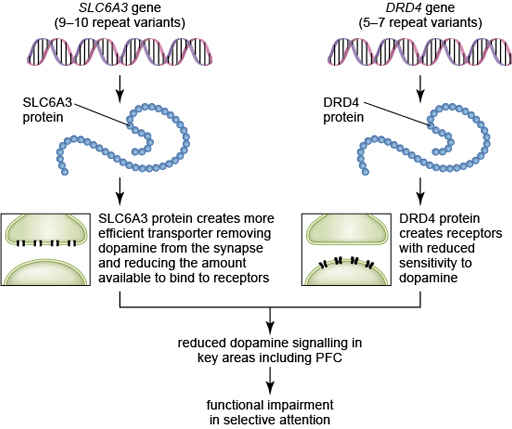
One gene that has been linked to ADHD is the gene that codes for (that is, carries the instructions for cells to build) the dopamine transporter channel. This gene is known as SLC6A3 and it can exist in several forms which differ according to the number of times a specific part of the genetic sequence within the gene is repeated (these are known as ‘repeats’).
Two specific forms of this gene have been associated with ADHD (Madras et al., 2002; Bonvicini et al., 2016):
- The nine-repeat form which has been associated with the condition in adults.
- The ten-repeat form which has been associated with ADHD in children.
In both cases it is thought that having these versions of the gene will result in higher levels of the dopamine transporter channel within the brain (Madras et al., 2002).
-
If more dopamine transporter channels are available, what impact will this have on removal of dopamine from the synapse?
-
If more dopamine transporter channels are available, dopamine will be removed from the synapse more effectively, meaning it has less chance to bind with receptors.
In addition to the transporter channel, a second candidate gene that may be of importance in ADHD codes for a specific type of dopamine receptor known as D4.
As with SLC6A3, the gene for the D4 receptor, known as DRD4, can exist in several different forms, again varying by the number of repeating sequences. Research suggests that having the longer version increases the risk of having ADHD (Kebir et al., 2009) by around 50%, while having the shorter version may confer a protective effect (Li et al., 2006), reducing the likelihood by around 10%.
The longer versions of DRD4 have been shown to alter dopamine receptor functioning and reduce a neuron’s response to dopamine.
-
If the receptor is less responsive to dopamine, what would this mean for the postsynaptic effect of dopamine binding to these receptors?
-
The normal effect of dopamine on the postsynaptic neuron would be subdued in the presence of the altered version of the receptor.
In terms of selective attention, the most important area affected by reduced dopamine signalling is the PFC. Figure 10 summarises the steps by which variants in the genes discussed here could result in impaired attention in ADHD. When viewing Figure 10, keep in mind that this shows how just two genes could impact on one area of attention and, therefore, the picture for ADHD as a whole would be much more complex.
As well as specific candidate genes relating to dopamine, one of the biggest indicators that ADHD may have a dopaminergic basis is that the main medications used to treat the condition, which were discovered long before the genetic methods became available, primarily target this neurotransmitter. You will learn more about these treatments in Section 2.2.3, but for now it is important to recognise that the various research studies identifying a role for dopamine in ADHD led to the dopamine theory of ADHD (Levy, 1991). This theory has changed slightly over the years but is still considered important and so shall be considered in the next section.