1.3 Isotopes
You will recall, way back in the nineteenth century, John Dalton popularised the idea of matter being composed of atoms. More specifically he proposed that the atoms of each element are identical, especially in mass.
The discovery of isotopes meant this description, whilst fine up to a point, doesn’t tell the whole story.
All atoms of the same element have identical atomic numbers. But sometimes the number of neutrons in the nucleus can vary for a particular element. This means you can have atoms of the same element with different mass numbers.
Atoms having identical numbers of protons but different numbers of neutrons are called isotopes.
Let's have another look at hydrogen.
You’ve seen hydrogen represented as , but there are also two isotopes of hydrogen which contain neutrons. These are deuterium having one proton and one neutron, and tritium with 1 proton plus two neutrons.
These have the chemical symbols: deuterium and tritium,
The key point to remember is because elements are defined by the number of protons not the number of neutrons even though they are isotopes they are still hydrogen atoms.
Bearing this in mind let’s now look at a ‘bigger atom’ and its isotopes.
If you take a lump of copper, all of its atoms have atomic number 29 meaning all their nuclei contain 29 protons (and 29 orbiting electrons).
However you will find some with 29 protons and 34 neutrons, and others with 29 protons and 36 neutrons in the nucleus, i.e. there are two isotopes (Figure 3).
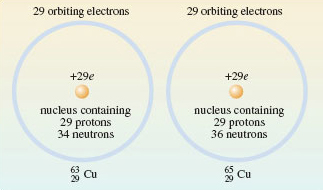
To reiterate, even though they have different numbers of neutrons, both of the atoms in Figure 3 are still copper.
Account for the mass numbers of the two copper isotopes shown in Figure 2.
Answer
Adding up the protons and neutrons we get: 29 + 34 = 63 and 29 + 36 = 65.
As shown in Figure 3, the two isotopes are written, and .
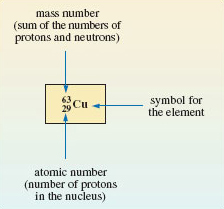
And just to clarify things further, the chemical symbol for one of the isotopes (the lighter one) is broken down into its components in Figure 4.