1.6 Models of the atom
Following his ground breaking experiment with alpha particles, Rutherford painted a picture of an atom being rather like a minute model of the earth orbiting the Sun, with electrons orbiting the atomic nucleus.
However, if electrons really did orbit the nucleus like planets, then they would in fact be constantly accelerating. Any charged object undergoing an acceleration would continuously emit electromagnetic radiation and lose energy. As a result, an electron orbiting in this way would rapidly spiral into the nucleus as its electrical energy reduced.
Clearly, this doesn’t happen, so electrons cannot really be simply orbiting the nucleus.
Consequently this model didn’t remain in vogue for too long.
By the 1920’s a branch of physics known as of quantum mechanics began to stamp its mark on the world of the chemist.
In a nutshell, quantum mechanics relates to the theory of matter on extremely small scales, and tells us that electrons in atoms cannot have well defined orbits but must be described in terms of probabilities.
Regions of space within an atom where electrons are highly likely to be found are known as orbitals.
So to reiterate this key point; quantum theory tells you that you can’t say exactly where an electron in an atom will be at any particular moment, you can only speak about the probability of finding an electron at a particular point.
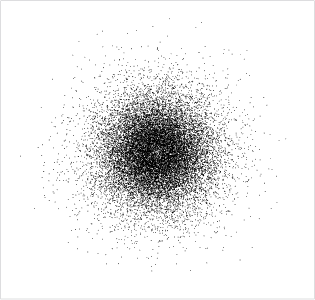
Figure 5 is a depiction of an electron in a hydrogen atom; the areas where the dots are closer together are those where the electron is more likely to be found, and overall this region of space is spherical.
So taking all the dots together, representing all the possible positions of the electron, you get a kind of ‘fuzzy cloud’ surrounding the nucleus.