4.4 The marine carbon cycle
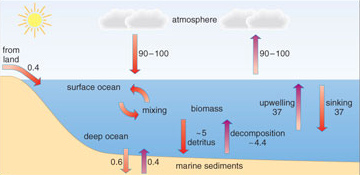
The ocean stores much more carbon than the terrestrial system (Figure 1.9). How is this marine carbon fixed into organic carbon within the sediments, and what are the main reasons for marine carbon fluxes? Figure 1.11 shows the rates of natural carbon exchange within the ocean and between the ocean and the atmosphere.
Question 11
From the data presented in Figure 1.11, is there a net movement of carbon into or out of the atmosphere from the ocean?
Answer
Figure 1.11 shows that 90-100 × 1012 kg of carbon flows each year from ocean surface waters into the atmosphere, while roughly the same amount of carbon moves from the atmosphere into the ocean: there is no net movement.
Carbon dioxide is constantly being exchanged between the atmosphere and the upper levels of the oceans, by physical and by chemical processes. At the base of the food chain that produces organic material in the oceans are the marine phytoplankton (microscopic water-borne plant life) which require dissolved CO2 for photosynthesis. The process also requires solar energy, so phytoplankton can live only in the sunlit upper parts of the ocean. Their photosynthesis releases oxygen, which dissolves in seawater. The productivity of phytoplankton depends on sunlight, temperature and supply of nutrients, and therefore varies geographically.
Carbon dioxide is more soluble in cold water than it is in warm water, so the concentration of dissolved CO2 tends to be higher in cold polar waters than in warm tropical waters. Cold, dense polar water sinks and flows under the influence of gravity along the ocean floor towards the Equator. It returns to the surface by upwellings at various places in the oceans, to supply nutrients and promote unusually high phytoplankton productivity there.
Zooplankton (water-borne animal life, mostly microscopic) and higher marine organisms consume these phytoplankton. The dead remains of phytoplankton, zooplankton and larger organisms sink through the water column, transferring carbon from the upper few hundred metres towards the ocean depths. However, little of this organic matter gets a chance to accumulate on the ocean floor. It provides food for filter feeders in deep water and on the ocean floor, and through them for predatory animals, and ultimately feeds the ubiquitous decomposers. All these organisms release CO2 back into solution through respiration. As Figure 1.11 shows, only a small proportion of the marine carbon cycle, 0.2 × 1012 kg yr−1 of carbon, is incorporated into marine sediments.
Question 12
Assume that 0.2 × 1012 kg yr−1 of carbon were incorporated into marine carbonates and fossil fuels in proportion to their present-day amounts (Figure 1.9). How long would it have taken to deposit all the carbon found in the current global store of POC, of which fossil fuels are a part?
Answer
We know from Figure 1.9 that the global carbonate store is some 4 × 1019 kg of carbon, and that the POC store is some 1019 kg of carbon, a total of 5 × 1019 kg of carbon. If carbon were deposited into each reservoir in proportion, the rate of deposition of carbon into POC would be:

At this rate, it would have taken 1 × 1019 kg/0.04 × 1012 kg yr−1, which is 2.5 × 108 years, i.e. 250 million years to deposit enough carbon to form the global POC store, which includes fossil fuels.
However, not all fossil fuel formed in the marine carbon cycle; much of it formed on land (Section 4.3). The preservation of organic material in sediments depends not only on the supply of dead organisms, but also on anoxic chemical conditions where they accumulate.
