2.1 Natural organic material
Much of the natural organic material found as a pollutant in water comes from domestic sewage and the effluents of farms and food-processing industries. Farm waste can be especially polluting and is an important EU issue: cattle slurry is up to 100 times as polluting per cubic metre as domestic sewage, and silage effluent is up to 200 times as polluting (Figure 5). Natural organic material consists of carbohydrates, proteins and fats, plus a number of other substances in lesser amounts. These are biodegradable; that is, they can be broken down by bacteria and other organisms into relatively harmless end-products. If sufficient oxygen is present in the water, aerobic bacteria (oxygen-using bacteria) feed on the organic material, using oxygen dissolved in the water. The polluting material is converted into water, carbon dioxide (CO2), nitrates (NO3-), sulphates (SO42-) and phosphates (PO43-). This process can continue as long as the bacteria can get enough oxygen from the water.

The oxygen concentration of unpolluted fresh water is around 10 mg l-1. The actual concentration depends partly on the rate at which oxygen is supplied by aquatic plants through photosynthesis and partly on the rate at which it is dissolved from the air—more oxygen is dissolved where there is a proportionately large surface area of water in contact with the air, such as in shallow ponds or turbulent rivers. Another important influence on oxygen concentration is temperature. The higher the temperature, the lower the amount of dissolved oxygen in the water: at 10 °C the concentration is about 11 mg l-1, falling to about 9mg l-1 at 20°C.
If excessive amounts of natural organic materials are discharged into a body of water, the demands of the bacteria feeding on it exceed the rate at which the oxygen can be replenished, and the oxygen concentration falls. This brings about a reduction in aquatic life; many animals in the water will die as the oxygen concentration decreases, and few plants thrive when organic pollution is severe. Trout and salmon die when the oxygen concentration falls below 3 mg l-1, and aerobic bacteria will not survive at concentrations below about 0.5 mg l-1.
At this point the decomposition of the polluting organic material is taken over by anaerobic bacteria (bacteria that exist in the absence of oxygen). These bacteria reduce the organic material to a different set of end-products — hydrogen sulphide (H2S), ammonia (NH3) and methane (CH4). They give the water a foul smell and indicate severe pollution.
The biochemical oxygen demand (BOD) is a measure of how much natural organic material—sewage, sewage effluent or industrial effluent —is present in a body of water. BOD is defined as the amount of oxygen taken up by microorganisms (principally bacteria) in decomposing the organic material in a water sample stored in darkness for 5 days at 20°C. Water with a high BOD has a low level of dissolved oxygen.
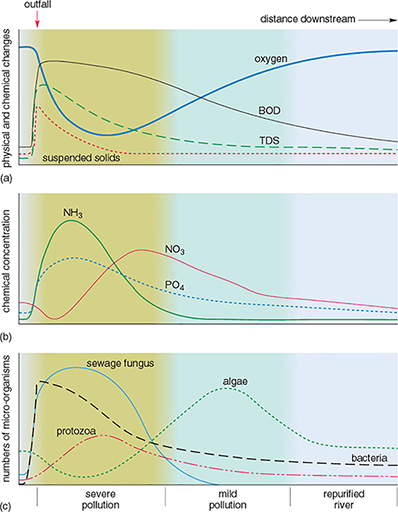
Rivers have some capacity for self-purification after pollution by biodegradable, natural organic materials. Figure 6 shows the effects of pollution downstream from a sewage outfall on one particular river.
Activity 3
Downstream from the sewage outfall, the dissolved oxygen in the water rapidly decreases and then gradually increases again (Figure 6a). How can this be explained?
How do the concentrations of ammonia (NH3) in Figure 6b vary?
Which organisms appear to be most able to tolerate the pollution (Figure 6c)?
What is the most likely explanation for the changes in the distribution of the organisms downstream of the sewage outfall?
Answer
The sudden decrease in oxygen a short distance downstream from the sewage outfall is caused by the oxidation of organic material, as shown by the increase in oxygen demand (BOD) just below the sewage outfall. After this, the water is slowly reoxygenated from the atmosphere and from aquatic plants, as the algal population increases.
Ammonia (NH3) concentrations increase after sewage enters the river, then gradually decrease downstream. (The increase is due to nitrogen-containing compounds being decomposed by anaerobic bacteria. The values decrease downstream as the ammoniacal compounds are oxidised, giving an increase in nitrate (NO3) concentrations.)
Bacteria, protozoa and sewage fungus appear to be most able to tolerate the pollution. These organisms are most abundant in the polluted parts of the river.
Organisms that tolerate low levels of dissolved oxygen are able to breed and thrive in the parts of the river closest to the sewage outfall. They have little competition and few predators. As the level of dissolved oxygen increases downstream, the less tolerant species are able to exist and may compete with others also able to survive there. As higher levels of dissolved oxygen are restored (that is, the river is self-purified), a more balanced aquatic ecosystem becomes established.
