2.5 What is water made of?
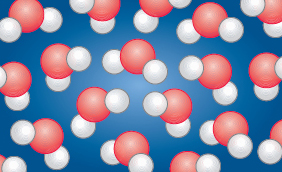
The size of a water droplet may seem very small but in terms of the scale of scientific measurement it is relatively large. You already know that water is made up of molecules so now consider a water droplet more closely to see what water molecules are made up of. If you could magnify a water droplet until it no longer has a smooth surface, you would see something similar to that shown in Figure 6. The spheres shown in the diagram are about 10−10 m in size and are called atoms.
One way of trying to visualise the size of an atom within a water droplet is to imagine a droplet magnified to the size of the Earth; an atom would be roughly the size of a tennis ball.
Figure 6 illustrates an important aspect of water, namely that it is made of water molecules, and that each molecule is made up of two types of atom: hydrogen atoms (shown as small white spheres) and oxygen atoms (shown as larger red spheres). Atoms are the basic building blocks of all material, whether the material is natural, such as rocks, plants and animals, or synthetic, such as plastic.
Question 3
How many hydrogen atoms are there compared with oxygen atoms in Figure 6?
Answer
There are two hydrogen atoms to every oxygen atom.
Question 4
How many hydrogen atoms and how many oxygen atoms are there in one molecule of water?
Answer
Two hydrogen atoms are joined to one oxygen atom to make one molecule of water.
Atoms are extremely small - about 10−10 m in diameter. Because they are so small, a model is needed to represent them and, moreover, to show how they are linked together to form molecules. Scientists use the term 'model' to mean any method of representing some structure or idea, so you won't be surprised to learn that there is more than one way of representing a water molecule. Figure 6 is one version, but there are others. If you are familiar with the children's building blocks called Lego®, you may find that a model based on this is helpful (seeSection 2.6).
