3.2 The agreement to protect the ozone layer
After a decade of controversy about the possible effects of CFCs, in 1985 British scientists discovered over the Antarctic a quite unexpected 'hole' in the ozone layer which was the size of the USA. This helped to galvanise the international community into action (though some who took part in the negotiations claim it played little part). By 1987 the first international agreement to control substances damaging to the ozone layer, the Montreal Protocol, was established. Interestingly the agreement was reached before there was evidence to link the ozone hole with CFCs and without any definitive examples of damage to humans or ecosystems.
The agreement has been rightly hailed as ground-breaking: by weighing current political and economic pressures against uncertain long-term hazards it was the first successful international agreement to incorporate the 'precautionary principle'. Later events have shown the wisdom of this approach. Each subsequent review of the agreement has tightened up the control of substances contributing to ozone depletion, as the scientific evidence of their effects became clearer, and frequently more alarming.
In industrialised countries, the production of CFCs and their use in new appliances has been banned since 1996, apart from critical uses, as in asthma inhalers, where no safe replacement has been found. This has resulted in global CFC production (weighted for ozone depletion impact) dropping by nearly 90 per cent from its peak in 1988 to 2000 (Figure 3).
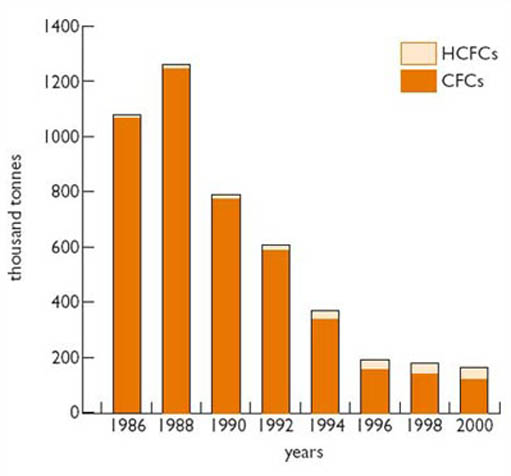
Levels of CFCs and other ozone-depleting chemicals peaked in 1994 in the lower atmosphere and by 2000 in the stratosphere, but will remain dangerously high for many decades because of their persistence. That is not quite the end of the story. Industrialising countries such as China, Brazil and India now account for almost all remaining CFC production (Figure 3). This group were exempted from the 1996 ban but agreed to freeze production in 1999 and reduce it incrementally to zero between 2005 and 2010. There remain problems with the safe disposal of CFCs in current use and illegal smuggling of chemicals from countries still in production. But areas of non-compliance with the agreement are likely to be of less practical significance than one of its compromises. It was only accepted by some manufacturers on condition they be allowed to use 'transitional' compounds for some purposes, to give them time to find and develop suitable long-term substitutes. The main family of transitional compounds (which are known still to damage to the environment) are called HCFCs, but for this discussion I will also include another group known as HFCs. Both groups are described in Box 1, which also lists some of their properties which can affect the environment.
SAQ 1
You are not expected to be familiar with the chemistry of CFCs and their substitutes, but Box 1 should help you to identify their potential for environmental damage. Read through it now then answer the following question.
What possible environmental problems could arise from using the two transitional compounds HFC 134a and HCFC141 b?
Answer
Although you have not been given any specific information about HCFC141 b and HFC 134a, you can expect each to have the properties described for the chemical family it belongs to.
As can be seen in Figure 3, which compares the ozone-depleting potential of HCFCs to CFCs, HCFCs including HCFC 141 b still have the potential to damage the ozone layer, though much less so than most CFCs. (In fact, molecule for molecule, its potential is about 10 per cent that of CFCs and this makes it the most damaging of the commonly used HCFCs.) HCFC 141b is also a powerful greenhouse gas. Its release in significant quantities could contribute both to ozone loss and global climate change.
HFC 134a does not have the potential to harm to the ozone layer, but is a very powerful greenhouse gas. Its release in significant quantities could contribute to global climate change.
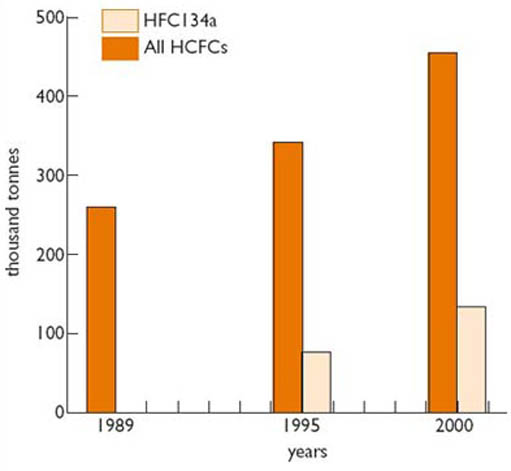
The refrigeration industry is a major user of both HFCs and HCFCs. Two compounds in particular have been favoured by them: HFC134a, as a refrigerant, and HCFC141b, as a foam blowing agent used in making insulation. Manufacturers have emphasised the desirable side of their properties, in particular their safety and effectiveness compared with alternatives, and, according to some pressure groups, have been reluctant to consider any other options. They moved quickly into production of these substitutes, which grew significantly in the late 1980s and 1990s, as illustrated in Figure 4. HCFC production peaked in the late 1990s and is now declining, while HFC continues its rapid rise in output, reaching 160 000 tonnes in 2002. The decline in CFCs in the 1990s (Figure 3), used mostly for refrigeration and foam blowing, has been mirrored by a corresponding rise in its substitutes.
Box 1: The environmental effects of CFCs and their substitutes
CFCs
CFCs or chlorofluorocarbons (compounds of chlorine, fluorine and carbon): These are highly stable molecules, with typical lifetimes in the atmosphere of between fifty and a hundred years. The most common compounds contain two or three chlorine atoms.
CFC substitutes
The two main families of chemical compounds developed as substitutes for CFCs, HCFCs and HFCs, are interim or transitional compounds, to be used for a limited time until more environmentally benign alternatives can be found and developed. The two properties of CFCs that give them the potential to damage the ozone layer are their stability and the number of chlorine atoms they contain. In the substitute compounds some or all of the chlorine atoms are replaced by hydrogen atoms. This has the effect both of reducing the length of time they persist in the atmosphere, so less of the compound eventually reaches the stratosphere, and, once it does, of reducing the amount of chlorine available for release to threaten the ozone layer.
HCFCs or hydrochlorofluorocarbons (compounds of hydrogen, chlorine, fluorine and carbon): In this family one or more of the chlorine atoms has been replaced by hydrogen atoms. This produces a less stable molecule that has a shorter lifetime in the atmosphere, typically about twenty years. Their potential for damage to the ozone layer is between a tenth to a twentieth that of CFCs.
HFCs or hydrofluorocarbons (compounds of hydrogen, fluorine and carbon): All the chlorine is replaced by hydrogen and thus HFCs are not damaging to ozone. They have similar lifetimes to those of HCFCs.
All of these three families of compounds, however, are also very potent greenhouse gases. Typically, measured over a lifetime of 100 years, each molecule of HCFC or HFC has the same effect as approximately one thousand molecules of carbon dioxide, and will thus contribute strongly to global climate change.
Some hydrocarbons (compounds only of hydrogen and carbon), for example, propane and isobutane, are now increasingly being used in refrigeration as a 'greener' alternative to both the CFCs and their transitional replacements. These, too, are greenhouse gases, with each molecule having approximately ten times the effect of a molecule of carbon dioxide. While this is still significant, it is considerably less than the effect of HCFCs or HFCs.
