4.5 Global climate change
I would like to turn now to the possible consequences of our use of energy for global climate change. Our pattern of energy use relies heavily on burning carbon-based fossil fuels, releasing carbon dioxide which spreads evenly around the globe and builds up slowly in the atmosphere. Carbon dioxide is a greenhouse gas, which means that it has the potential both to warm the atmosphere and to change our global climate. It is not the only greenhouse gas but is the most important of those emitted through human activity – accounting for over half of the human induced greenhouse effect. This effect and its relation to global warming is explained in Box 2 which also discusses CFCs and the ozone hole over the Antarctic: these two effects – ozone loss and global warming – are often confused.
Box 2: What exactly is 'global warming'?
(Many of the terms in this box are defined in the Glossary.)
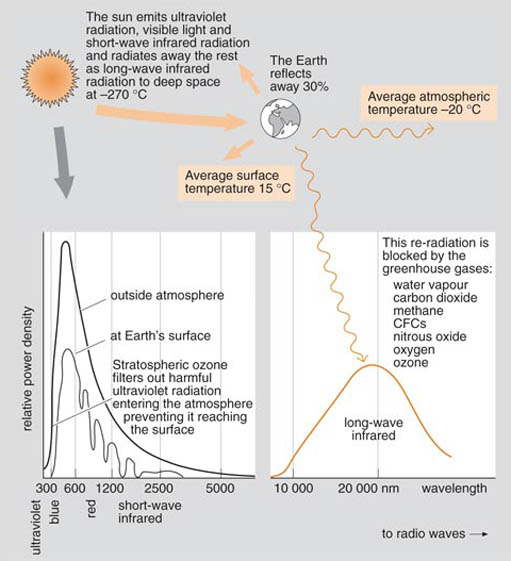
The Earth sits in space in a temperature balance between the searing hot surface of the Sun (about 6000°C) and the bitter cold of deep space (around −270°C). Life on Earth has adapted to its average surface temperature of about 15°C.
The Sun emits a range of wavelengths of radiation, ranging from ultraviolet light with wavelengths shorter than 350 nanometres (nano=10−9), through visible light to short-wave infrared radiation with wavelengths of 1000−2000 nanometres (Figure 9). Of this incident energy, about 30 per cent is simply reflected back into space. The remainder is absorbed by the atmosphere and the Earth's surface. Eventually, this energy is also re-radiated back out to space, but this time as long-wave infrared radiation, with wavelengths of over 10,000 nanometres. We tend to forget about this outgoing radiation until we encounter a ground frost on a cold clear night as the heat radiates first to the cold upper atmosphere (at around −20°C) and then out into space.
This re-radiation is partially blocked by a number of so-called greenhouse gases in the lower atmosphere which are to varying degrees opaque to long-wave infrared radiation. The fact that they do this means that the Earth is about 35°C warmer than it would be without them (hence the 'greenhouse'). The most important of these is water vapour. A cloudy sky is very good at preventing a ground frost on a winter's night. Other naturally occurring greenhouse gases are carbon dioxide, methane, ozone, and nitrous oxide. Some of these are also produced by human activities:
carbon dioxide from the burning of fossil fuels and forests;
methane produced by cattle, rice paddy fields, decomposing rubbish and leakage from natural gas pipelines;
tropospheric ozone produced by traffic pollution;
nitrous oxide produced from agricultural fertilisers and the manufacture of nylon;
CFCs (which do not occur naturally at all).
The debate about climate change (see Section 3.4 below and Box 3) is concerned with the enhanced greenhouse effect due to gases added to the atmosphere by human activity. More carbon dioxide will increase the blocking of outgoing infrared radiation and require a rise in global temperature to restore the equilibrium.
But don't CFCs just cause the ozone hole?
Many people confuse the ozone hole with global warming, because two of the key players, CFCs and ozone, feature in both stories. Even worse, ozone turns up again in urban smog. Let's run through the differences.
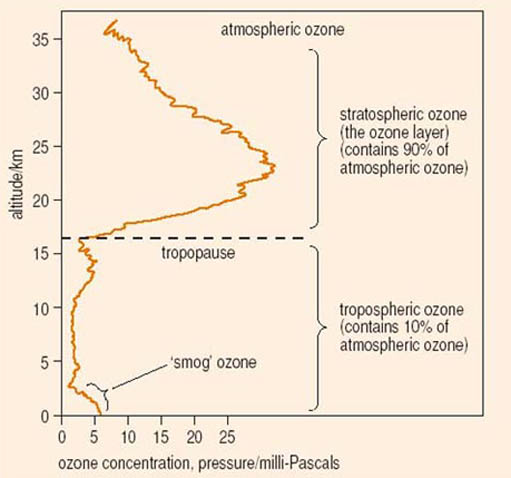
Most of the ozone in the atmosphere is contained in the stratosphere. That is a very cold region extending from about 15 to 50 kilometres above the Earth's surface (jet airliners mostly fly at under 10 kilometres). It is the stratospheric ozone that mainly filters out the harmful ultraviolet rays of the Sun (Figure 10). CFCs attack and destroy this ozone in the very cold stratospheric conditions over the Earth's poles, creating the'ozone hole'.
However, CFCs also strongly absorb long-wave infrared radiation, making them important anthropogenic greenhouse gases. This effect takes place all over the atmosphere and all over the globe. This has made it doubly necessary to phase them out.
Is ozone a good gas to have around? Obviously having plenty in the stratosphere keeps the Sun's ultraviolet rays at bay, but down on the Earth's surface it acts as a powerful oxidising agent, destroying vegetation. Industrially it is commonly used as a bleach and as a disinfectant for swimming pool water. 'Tropospheric' or low-level ozone is a particularly toxic ingredient of the 'photochemical smog' that occurs over cities such as Los Angeles, Mexico City and Athens. Reducing this kind of pollution is a key concern in the search for cleaner methods of transport.
Exercise 3
The aim of this exercise is to help sort out any confusion between global warming and ozone loss.
List in turn the gases associated with human-induced global warming and ozone loss, and identify the main gas or family of gases involved.
Identify (but do not attempt to explain) the chief mechanism thought to cause human-induced global warming and ozone loss respectively.
State in which part of the atmosphere each effect mainly takes place.
Finally, which gases are most likely to be involved in both effects?
Discussion
The main gases associated with human-induced global warming are carbon dioxide, methane, nitrous oxide, tropospheric or ësmog' ozone and CFCs. Carbon dioxide is the most important of these. The most important family of gases involved in ozone loss is that of the CFCs. The other family mentioned in Box 1 is the HCFCs. In both cases you may know of other gases that have been identified. Note that we have not included water vapour here which of course occurs naturally, but is not emitted in significant amounts as a result of human activity.
The mechanism thought to cause human induced global warming is the greenhouse effect (strictly speaking the enhanced greenhouse effect), which is why the gases listed in the first part of (a) are called greenhouse gases. Ozone in the stratosphere is destroyed in certain circumstance by CFCs, particularly when the temperatures are very low. The details are extremely complex and we will leave it at that.
The greenhouse effect mostly takes place in the lower part of the atmosphere, or troposphere, which contains most of our atmosphere and most of the greenhouse gases. Ozone loss refers to the destruction of ozone in the stratosphere. Again the reason is the same – most of the ozone occurs in the stratosphere.
The gases most likely to cause confusion are, first, the CFCs, which contribute both to global warming and ozone loss, and secondly, ozone, which occurs in the stratosphere where it serves a vital purpose and is the 'victim' of depletion by CFCs. Ozone also occurs in the lower atmosphere where it forms summer smogs and is also a powerful greenhouse gas. There are other interactions – global warming and ozone loss are both very complex phenomena, but once again we had best leave it at that.
There is also considerable uncertainty in making the links between increased levels of carbon dioxide in the atmosphere and forecasts of global warming and climate change. For example, there are difficulties in modelling some key aspects of global climate, such as the interactions between oceans and the atmosphere and the behaviour of clouds. There are also problems in achieving a realistic forecast of human population and economic activity far into the future to arrive at the projections of future carbon dioxide emissions on which the models rely. Indeed, scientists are not agreed on the extent to which we are currently experiencing global climate change. (There is also a minority who still question the reality of greenhouse gases as a significant cause of climate change, though many of their arguments have now been answered.)
What this means is that there exists a major threat of change to our climate, but significant uncertainty as to its extent. In spite of the inability to provide precise forecasts, the great majority of scientists and policy makers decided at the 1992 Earth Summit in Rio de Janiero to act on this issue, and made their first, tentative agreement five years later at Kyoto. Box 3 indicates some of the likely impacts of climate change.
