3.4.2 Periodic arousal
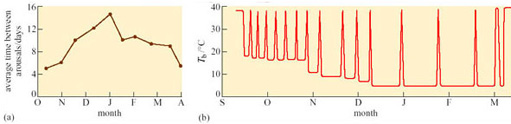
All mammalian hibernators arouse periodically. The frequency of the arousal and the length of the euthermic periods between bouts of hibernation vary widely with species, among individuals, and with the time of year (e.g. in deep hibernators, the larger species seem to have longer periods of wakefulness than the smaller ones). The arctic marmot (Marmosa caligata), whose heart rate recording is shown in Figure 13, aroused from hibernation every 2–3 days and remained euthermic for 3–4 days at a time. Figure 16a shows the average number of days between successive arousals of golden-mantled ground squirrels at various times in one hibernation season and Figure 16b shows the frequency of arousals in a Richardson's ground squirrel (Spermophilus richardsonii).
Evidence points to the likelihood that the internal mechanisms which set circadian rhythms do not operate during deep hibernation. But in studies on hibernation in European ground squirrels kept under conditions as close to the natural habitat as possible, continuous recording of temperature using implanted thermal dataloggers showed that entry and arousal from torpor was synchronized to the time of day in some animals. All animals entered torpor in the afternoon and some even aroused at the same time. Circadian temperature fluctuations occurred at higher temperatures during entry and arousal phases.
What could be the function of periodic arousals? Some hibernators, such as hamsters, store food for the winter in their nests or burrows, and eat during the periods of arousal. Bats arouse to drink on mild nights. For those species that only metabolize fat from their stores during hibernation, the reason for arousal is not so obvious, particularly as the energy expended in a single arousal lasting a few hours can equal that used in 10 days of hibernation. In the arousing golden hamster (Mesocricetus auratus), changes in oxygen consumption and temperature in various parts of the body are rapid and extensive (Figure 17).
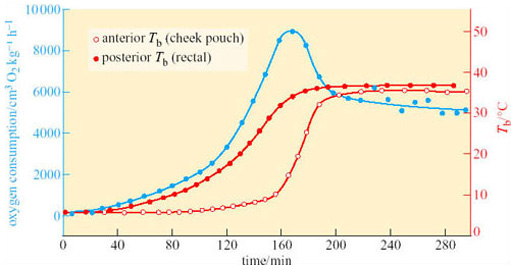
At the start of arousal with an ambient temperature of 5° C, oxygen consumption is 60–80 cm3 kg−1 h−1. Within 3 hours this rate rises 100-fold, to a level comparable to that of violent exercise. At the start of arousal, cheek pouch temperature is virtually the same as that of the rectum but you can see that, as arousal progresses, the cheek pouch temperature rises more rapidly and there is a difference of more than 20° C by 160 minutes. A short time later, oxygen consumption reaches its peak and declines rapidly, whereas cheek pouch and rectal temperatures attain the euthermic level. In some species, for example many ground squirrels, the process of arousal may even be much faster than it is in the hamster (Figure 18).
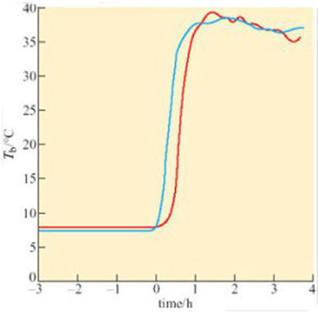
A difference between anterior and posterior T b during arousal is seen in virtually all hibernators, including bats. Since the end of the 19th century, it has been known that there are profound circulatory adjustments during hibernation. By using a radio-opaque dye and X-ray equipment, it is possible to show that blood flow to the posterior region of the golden hamster is restricted during hibernation, but increases in the forelimbs, heart, diaphragm, thorax and deposits of Brown Adipose Tissue (BAT) during the initial stages of arousal. Figure 19 shows various measures, blood pressure, heart rate, and rectal and heart temperatures, during arousal of a 13-lined ground squirrel (Spermophilus tridecemlineatus) (Lyman and O'Brien, 1960).
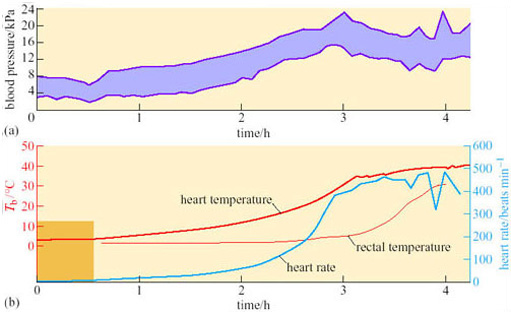
Peripheral vasoconstriction, and thus the resistance to blood flow, appears to lessen at the start of arousal, indicating vasodilation, but then rises rapidly while the heart rate is also increasing: as a consequence the rapidly beating heart is working against a high blood pressure. In these circumstances, although the heart may be an inefficient pump it is a good source of heat as the animal warms up. As rectal temperature increases rapidly, the blood pressure starts to decline, associated with a decrease in peripheral resistance, probably due to a sudden vasodilation in the posterior regions of the body is thought to be responsible. Evidently, vasomotor changes are important in arousal as well as in entry to torpor.
Hibernators arouse without recourse to external heat, so what is the source of the heat required? The violent shivering that accompanies some stages of arousal suggests that contraction of skeletal muscle is important but even animals in which skeletal muscle activity has been inhibited by curare (which blocks transmission at neuromuscular junctions) can re-warm.
