4.4 Cell survival mechanisms
Physical damage is not the only danger that faces cells recovering from low temperatures in the absence of oxygen (due to a 90% drop in blood flow to the brain) and energy supplies. A universal sign of recovery from such conditions is the production of reactive oxygen species (ROS) (Box 4). The electron transfer chain that participates in the formation of water from oxygen in mitochondrial respiration can also be used in the production of the free radical superoxide, sometimes called ‘singlet oxygen’ because the molecule contains an extra unpaired electron. These short-lived molecules can initiate spontaneous chain reactions through electron transfer leading to the generation of highly reactive hydroxyl free radicals. They then react with free fatty acids forming the compounds malonaldehyde and 4-hydroxynonenal, which cause damage to proteins and nucleic acids, eventually leading to cell death. There are two adaptive mechanisms which hibernating vertebrates have adopted to counter the potentially toxic consequences of a surge in oxygen supply on arousal.
Box 4: Generation of reactive oxygen species
Normal respiratory pathway:
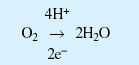
Generation of superoxide:

Generation of hydroxide free radicals:
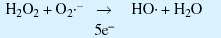
Generation of lipid peroxides from hydroxide free radicals:

Generation of reactive species that damage proteins and nucleic acids:

First, the concentration of a number of ROS-neutralizing compounds such as vitamin C (ascorbic acid) and glutathione, and the enzyme superoxide dismutase, increases on arousal from hibernation. Neutralizing compounds are ‘scavengers’ of lone electrons present in superoxide and hydroxyl free radicals, whilst an increase in the activity of superoxide dismutase results in the conversion of all the superoxide to hydrogen peroxide and hence reduced formation of the hydroxyl free radical. In the ground squirrel, circulating levels of ascorbic acid increase by up to five times. Continuous measurements of blood ascorbate in arctic ground squirrels during arousal show that the levels of anti-oxidant start to decrease at the peak level of oxygen consumption, indicating that ascorbate is being distributed to respiring tissues to counter oxidative damage.
Secondly, proteins that prevent the sequence of events leading from ROS damage to cell death are activated. In the 3-lined ground squirrel (Lariscus insignis), cells lining the intestine are particularly vulnerable to ROS damage as they adapt to the absence of dietary nutrients. Lipid peroxides are one of the end-products of superoxide activity and their levels increase during entry into, and during, the early phase of a torpor bout. Their formation is accompanied by a substantial increase in biosynthesis of a protein known as NFκB. The protein is a gene regulator that is only stimulated in affected cells by redox reactions which accompany the generation of superoxide. No stimulation is seen in WAT where no lipid peroxidation is measured. NFκB activates genes that lead to cell death by preventing metabolic pathways initiated in the mitochondria. A hibernation induction trigger (HIT) circulates in the plasma of hibernating mammals ( and is believed to protect cells from death by activating similar protective genes. This area of hibernation research, as you might expect, is of considerable interest to medical researchers seeking the means to protect victims of cerebral ischemia and stroke, characterized by loss of blood flow very similar to that experienced by hibernators, from long-term tissue damage.
