5.6 The importance of size and habitat
The use of hibernation to gain energetic advantage must be weighed against a number of considerations, particularly animal size and behaviour, biogeographic distribution and habitat. Small animals, which can carry less fat and have a higher surface area to volume ratio and BMR, are more likely to lose energy as heat and in maintaining life functions if they do not use hypothermic strategies in winter. Few hibernating mammals have a total body mass greater than 5 kg. Indeed, in large animals there may be a cost advantage in not hibernating as less energy is likely to be needed to see them through the winter in a prolonged state of fasting close to a thermoneutral T a, than in hibernation with re-warming. For example, an adult bear's re-warming energy cost is 105 greater than that of a mouse and the energy required for re-warming is equivalent to the energy needed to remain euthermic for nearly 3 days (compared to 3 hours in the mouse).
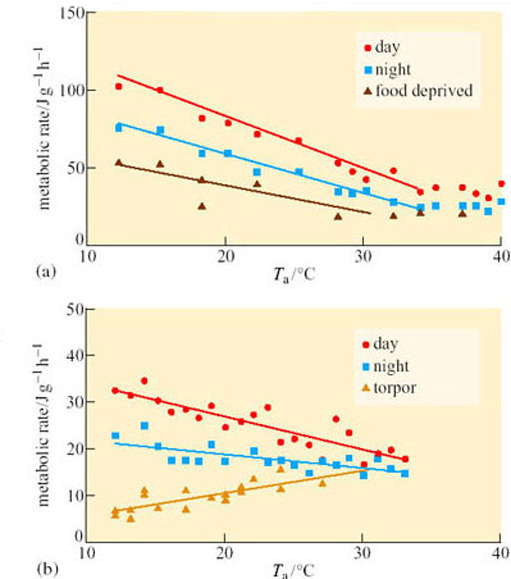
The importance of size is balanced by the importance of the habitat, and by the ability of animals to evolve their thermoregulatory strategy to match ecological energy demands, enables them to diversify just as much as their foraging and locomotion techniques. Pigeons and doves (Columbidae) for example, belong to a large (300 species) family of birds that shows remarkable ecological diversity. Hypothermic strategies are used by species whose adults range in mass from 35 to 800 g. An implanted temperature-sensitive transmitter was used to measure T b, and a flow-through respirometer for V O2 and V CO2. Cloven-feathered doves (Drepanoptila holosericea) are fruit-eating birds from the island of New Caledonia, and the effects of food on the energy metabolism of the only captive pair in the world were compared with the effects on grain-eating African Namaqua doves (Oena capensis) which are adapted to arid desert habitats. Namaqua doves show shallow torpor in response to food deprivation, with T b falling to 30–37° C: there was a fluctuation of T b with T a, but a metabolic defence at T a below 25° C. This regulation is reflected by the increase of metabolic rate at low T a (Figure 43a). This exercise saves them around 10% of their daily energy requirements. In contrast, cloven-feathered doves (Figure 32b) show a more pronounced onset of deep torpor (with T b falling to between 24 and 30° C), induced by darkness at T a lower than 15° C, and leading to a substantial reduction in metabolic rate of up to 62%. However, this torpor only lasts up to 3 hours as the process of cooling is also several hours in duration. Total daily energy consumption is reduced by a comparable amount (10–15%), including the cost of re-warming. The very different form of thermoregulation in the cloven-feathered dove therefore, has very little energy budget advantage and may instead be an adaptation to its island habitat together with its fruit diet, that may make it difficult to find food at certain times of the year.
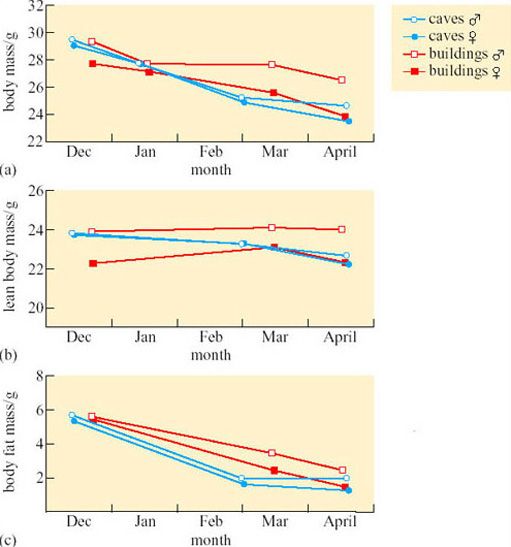
Further evidence that energy budgeting varies with habitat comes from work on mouse-eared bats (Myotis myotis) (Koteja, 2001). Using a non-invasive method called Total Body Electric Conductivity (TOBEC), lean body mass and fat content were measured in hibernating animals either in buildings or caves in Poland (Figure 33). Although food availability was similar in each habitat, fat content was reduced from 19 to 6% of total body mass between December and April in both males and females. Calculations showed that the bats need about 4.9 g of fat (191 kJ) to sustain a 165-day hibernation. However, the rate of fat usage varied considerably between different sites and at different phases of hibernation. Although the average amount of fat remaining in April would be sufficient to support at least six more weeks of hibernation, the level of reserves was close to zero in some individuals.
A model has recently been drawn up to predict the relative energetic advantages of hibernation in little brown bats (Myotis lucifugus; Figure 9) living in different latitudes. T a at each latitude influences total winter energy requirements. Hibernation is only likely to confer bioenergetic advantages within a fairly narrow range of winter durations for animals living at favourable hibernaculum temperatures. As it turns out, M. lucifugus lives no further north than predicted by the model and so hibernation energetics could be the most important factor governing its geographic range, of this species at least. It follows that the consequence of global warming might be a northward expansion of the species within only two or three generations.
