3.2 Integration of anatomy and behaviour with biochemical and physiological strategies in evaders
We know from Section 2.3 that small desert rodents remain cool by staying in their burrows for all or part of the day. Kangaroo rats (Dipodomys spp.; see Figure 20 in Section 2.3) depend on metabolic water as there is little or no water available in their diet of seeds. Kangaroo rats appear to be ill-adapted for life in a desert; like other rodents they neither sweat nor pant. Nevertheless, inside the burrow, they could lose water by evaporation from the lungs, which would be enhanced by T b being higher than burrow T a. As the water-carrying capacity of air increases with temperature, warm expired air contains more water than the cooler inhaled air.
However, the temperature of the exhaled air in kangaroo rats is lower than that of T b, and often close to T a (Figure 26). This is because the nasal passages (turbinates) of kangaroo rats are extremely narrow and convoluted and provide a temporal counter-current cooling system, which operates as a heat exchanger (Figure 27).
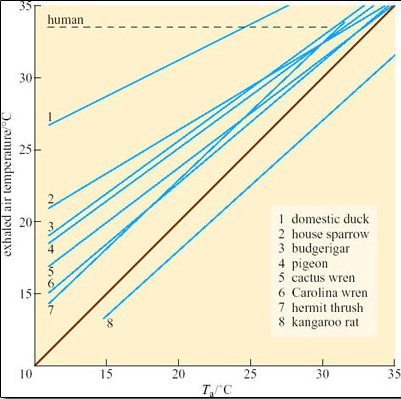
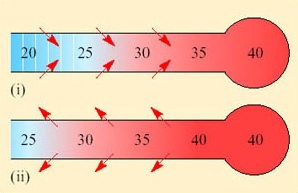
Both inspired and expired air pass over the same surface, the nasal mucosa. Air entering the nasal passages takes up both heat and moisture from the mucosa and is therefore both warmed and moistened before entering the lungs. In the short interval between inhalation and exhalation, the thermal gradient between nose and trachea is maintained. When air is exhaled from the lungs, initially its temperature is 37–38°C and it is humidified by heat and moisture derived from the warm tissues in the nasal passages, trachea and bronchi. As the exhaled air approaches the nasal passages, the temperature and vapour pressure gradients between the mucosa and the adjacent air are reversed and heat is lost from air to the mucosa. During cooling, water condenses on the mucosal surfaces. At the tip of the nose, the air is expired at ambient temperature, still saturated with water vapour, but because its temperature is reduced, it carries much less water. The efficiency of the heat and water exchanger reflects the large surface area to bore ratio of the nasal passages of a small animal like the kangaroo rat. When the kangaroo rat breathes air at 25 per cent relative humidity, the temperature of the expired air ranges from 31°C at T a 35°C to 13°C at T a 15°C. About 54 per cent of the water vapour derived from evaporation from the respiratory surfaces is thereby conserved at 30°C and 83 per cent at 15° C. In contrast, humans, with short wide nasal passages, cannot recover more than 16 per cent water vapour at T a ranging from 12 to 35°C.
The following material gives some important background on kidney function and to appreciate this you need to revise the concept of osmosis. Osmosis is the movement of water between two solutions which have different solute concentrations, and which are separated by a semi-permeable membrane. Water will move from the side that has lower solute concentration to the side that has the higher solute concentration. Osmolarity is an expression of the osmotic concentration of the solution. You may find texts where solutions with a high osmolarity are referred to as having a high osmotic pressure. You can think of this in terms of the pressure that would have to be applied to the solution on the side with a high solute concentration to prevent the movement of water by osmosis. The greater the solute concentration, the greater the pressure needed to prevent osmosis.
Kangaroo rats and other desert rodents, e.g. the Australian hopping mouse Notomys, conserve water by producing extremely hyperosmotic urine, on average 5500 mOsmol l−1 in Dipodomys and 9000 mOsmol l−1 in Notomys. Compare the osmolarity of the urine of Dipodomys with that of other mammalian species (Table 3), and note how small xeric mammals produce more highly concentrated urine than do species living in mesic habitats. Note also that large mammals living in xeric habitats, e.g. camels, do not produce urine as concentrated as that produced by small xeric mammals. Values for the net ratios of osmolarity for urine and plasma (U/P ratios) are provided to demonstrate the concentration of urine relative to that of the blood. Osmolarity is the concentration of solute particles, not the concentration of moles, although the two are related. For example, a solution containing 1 mol I−1 sodium chloride has an osmolarity of 2 Osmol I−1, because in solution, sodium chloride molecules break down into equal numbers of sodium and chloride ions. In contrast, molarity and osmolarity for a glucose solution are the same because glucose molecules remain intact in solution.
The ability of the kangaroo rat and other desert rodents to produce a hyper-concentrated urine is attributed to their possession of extremely long loops of Henle, which is often quoted as an extreme adaptation for life in parched deserts. But is the ability to produce a concentrated urine an ‘extreme adaptation’? Mammalian kidneys are effector organs that maintain the concentration of salts and excretory products, especially urea, in the blood within very narrow limits.
| Mammal | Habitat | Urine concentration/mOsmol I−1 | U/P ratio |
|---|---|---|---|
| Small mammals | |||
| rat | mesic | 2900 | 9 |
| domestic cat | mesic | 3100 | 10 |
| kangaroo rat | xeric | 5500 | 16 |
| Large mammals | |||
| beaver | freshwater/land | 520 | 1.7 |
| human | mesic | 1400 | 4–5 |
| porpoise | marine | 1800 | 5 |
| eland | xeric | 1880 | 6 |
| camel | xeric | 2800 | 8 |
The mammalian kidney is a compact organ consisting of an outer dark cortex and an inner pale medulla (Figure 28). The kidney tissue is made up of nephrons, which are thin-walled tubules (not to scale in this figure). The nephrons are concentrated in areas known as pyramids. Ducts that collect the urine and transfer it to the ureter are located in the papilla areas.
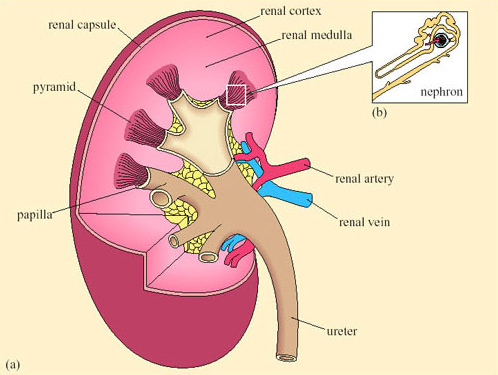
Each nephron begins with a cup-shaped structure, the Bowman's capsule. This encloses the glomerulus, a cluster of capillaries. (The Bowman's capsule and the glomerulus together are sometimes referred to as the Malpighian body.) Bowman's capsule opens into the coiled proximal convoluted tubule, which leads to the loop of Henle (Figure 29). There are two types of nephron, distinguished by the length of their loops of Henle.
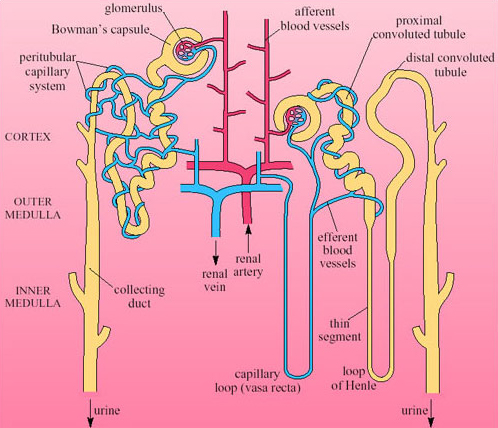
Cortical nephrons have short-reach loops that just penetrate the boundary between the inner and outer zones of the medulla. Juxtamedullary nephrons have long-reach loops that penetrate deep into the medulla. In humans about 15 per cent of nephrons are juxtamedullary and 85 per cent are cortical. Blood reaching the Bowman's capsule undergoes ultrafiltration. The blood pressure in the glomerular capillaries is high, and it is maintained by the pumping of the heart and the mechanical properties of the blood vessels. Consequently blood in the glomerulus is filtered through the basement membrane of the capsule. Blood cells and proteins remain in the blood, so that the filtrate that enters the nephron tubule has a similar composition to plasma minus its proteins and large lipids. Osmolarity of plasma and filtrate are the same, 300 mOsmol l−1.
As the filtrate travels through the nephron its composition undergoes considerable modification. The movements of sodium, chloride and water are summarised in Figure 30.
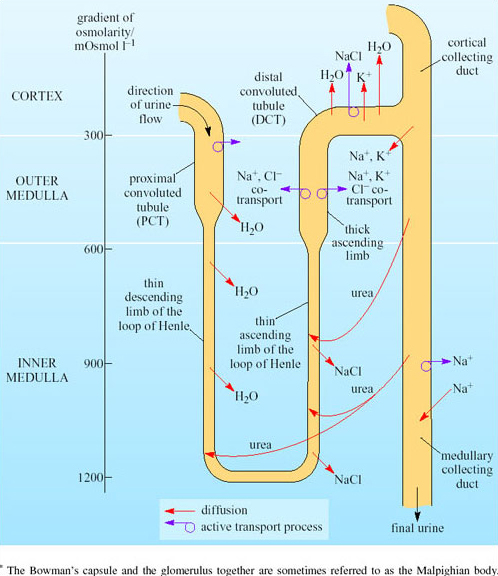
The values on the left in Figure 30 are the osmolarity of the interstitial tissue. You can see a gradient of osmolarity from 300 mOsmol l−1 in the cortex to 1200 mOsmol l−1 in the inner medulla. The fluid in the loop of Henle has about the same osmolarity as the fluids in the surrounding tissue. Movements from the tubules of Na+, K+, Cl−, urea and water occur as shown. Relatively small amounts of salt moving from the ascending limb to the interstitial tissues will cause osmotic movement of water out of the descending limb. Due to the principles of counter-current multiplication, a small difference in the concentration between adjacent points on the two limbs leads to a large difference in concentration between the top and bottom of the loop. Since the collecting tubule is very permeable to urea, urea moves into the interstitial tissues. This will increase the osmolarity in the medulla. The thin ascending limb has some permeability for urea so you can view the collecting duct and ascending limb as ‘recycling’ urea. As urea is moving through the medulla, this increases the osmolarity in this region of the kidney.
The process begins in the proximal convoluted tubule (PCT), where the epithelial cells absorb much of the filtrate passing it back into the blood flowing in the surrounding vessels. Active transport of sodium out of the PCT epithelial cells into the interstitial tissues increases the osmolarity in the tissue. Thus water moves by osmosis out of the PCT. Movement of glucose, amino acids and water is coupled to movement of Na+ out of the tubules. The water permeability of the PCT is high because of the abundance of special membrane channel proteins, aquaporins, in the cell membrane. Permeability of PCT epithelial cells is relatively low for urea, so the 75 per cent reduction in fluid volume in the PCT results in a four-fold increase in urea concentration.
The counter-current system of loop of Henle concentrates the urine. Figure 30 shows that the hairpin-like loop of Henle lies between the proximal convoluted tubule and the distal convoluted tubule. Fluid entering the loop flows down the descending limb and then turns the corner, before flowing up the ascending limb. The loop of Henle functions as a counter-current multiplier system as a result of the opposing direction of fluid flow in the descending and ascending limbs. Although the filtered liquid flows into the descending limb of the loop of Henle first, we need to look at processes in the ascending limb so that we can understand what happens in the descending limb. In the ascending limb, sodium and chloride ions are reabsorbed into the medullary interstitial tissues, passively in the lower part of the limb and actively by means of Na+-K+ ATPase pumps in the thick upper part of the ascending limb. The active transport of Na+ out of the tubule cells creates low [Na+] and [Cl−] in the cell cytoplasm; this creates a concentration gradient drawing in Na+ and Cl− ions from the lumen of the tubule into the tubule epithelial cells via luminal membrane transport molecules in the upper part of the limb. Unlike the descending limb, the ascending limb is relatively impermeable to water, so little water follows the salt. The interstitial fluid of the medulla thereby becomes hyperosmotic compared with the fluid in the ascending limb. The apical membranes of the epithelial cells lining the descending loop of Henle have a very low permeability to ions and urea but a very high permeability to water. Water therefore diffuses out of the fluid in the tubule and into the epithelial cells and then into the interstitial fluid. Water diffuses out of the descending limb into the more concentrated interstitial fluid until the osmolarity between ascending and descending limbs is equal. As the ascending limb is continually pumping sodium and chloride ions, the concentration difference between it and the interstitial fluid is maintained. The osmolarity difference of 200 mOsmol l−1 is multiplied to 1400 mOsmol l−1 at the bend in the loop.
When the fluid reaches the distal convoluted tubule (DCT) its osmolarity has become reduced to just 100 mOsmol l−1. The fluid is diluted further in the DCT, where active transport in the epithelium removes more sodium and chloride from the tubular fluid into the epithelial cells. As the epithelial cell membranes are impermeable to water, the tubular fluid is hypo-osmotic by the time it reaches the cortical collecting duct.
As in the PCT, basal and apical membranes of the epithelial cells of the collecting ducts have aquaporins. Normally, these membrane channel proteins are configured to limit water reabsorption. If the blood osmolarity rises, antidiuretic hormone (ADH) is released from the posterior pituitary. This hormone acts directly on the aquaporins in the collecting duct epithelia so that the membrane channels are fully ‘opened’ and water moves by osmosis out into the interstitial tissues. This reduces the urine flow. If the blood osmolarity decreases, secretion of ADH stops and the membrane channels close, so water is retained in the collecting ducts. The blood vessels in the medulla, the vasa recta, are arranged as hairpin loops that run close to and parallel to the loops of Henle and collecting ducts. Water reabsorbed from the collecting ducts enters the blood capillaries and leaves the kidneys in venous blood, which maintains the concentration gradients in the medulla. As water is reabsorbed along the entire lengths of the medullary collecting ducts, fluid emerging from the medullary collecting ducts has the same osmolarity as the interstitial fluid around the bend of the loop of Henle at the bottom of the medulla. The longer the loop of Henle relative to the overall depth of the cortex, the higher is the osmolarity of the fluid in the bend. The kidney thereby retains as much water as possible, minimising loss of water during water shortage.
The relationship between the ability to concentrate urine and the length of the loops of Henle is not straightforward. There is no clear relationship between actual loop lengths and urine concentration in mammals. Average lengths of loops of Henle are not directly proportional to urine concentration when comparing large with small species of mammals. Notomys has a loop length of 5.2 mm and produces urine of up to 9000 mOsmol l−1 in contrast to the horse with a loop length of 36 mm producing urine of 1900 mOsmol l−1. How can we explain this data in relation to the hypothesis that the length of the loop of Henle does affect final urine concentration?
Small mammals have much higher mass-specific metabolic rates than large mammals. Compared with large mammals, the apical membranes in the kidney tubules of small mammal epithelial cells have more infoldings, increasing surface area for absorption.
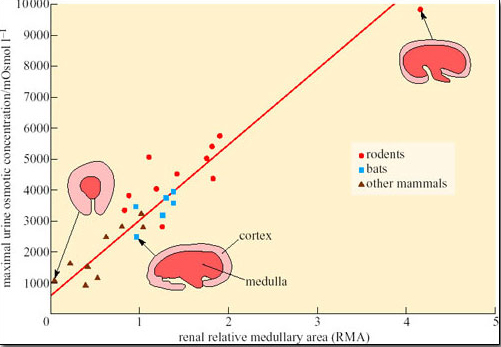
Increased metabolism will lead to more waste products and a greater demand on the filtration capacity of the kidneys. Therefore the number of nephrons must increase as body size increases, which in turn increases the relative amount of cortex (the area of the kidney where most of the nephron is located), at the expense of the medulla. The relative thickness of the medulla is related to urine-concentrating ability because the medulla contains the loops of Henle. Hence larger animals, even the camel, cannot produce urine as concentrated as that of smaller mammals, because their kidney medulla is relatively small compared with its cortex. Small mammals such as rodents and bats tend to have relatively thicker medullas than larger mammals, which can be correlated with their production of concentrated urine (Figure 31).
Activity 6
What conclusions could you draw from the data shown in Figure 31?
Answer
Rodents generally have kidneys with a larger medullary area and produce more concentrated urine than bats and other mammals.
The thicker medulla of small desert rodents could therefore be viewed as a desert adaptation superimposed on a basic body-size-dependent pattern. Most loops of Henle in desert rodents are of the juxtamedullary type, and the epithelial cells have densely packed mitochondria with more cristae per unit volume than a horse's loop of Henle.
Activity 7
What is the significance of the greater concentration of mitochondria and more cristae per unit volume of mitochondria in the epithelial cells of loops of Henle in desert rodents compared with those of the horse?
Answer
The greater numbers of mitochondria and cristae in epithelial cells of the loops of Henle of desert rodents suggest a higher capacity for ATP synthesis and therefore active transport of Na+ and Cl− ions in the kidney of desert rodents.
Conservation of water by the kidney is of crucial importance for the kangaroo rat, which does not drink and can obtain water only from catabolism.
Other desert rodents obtain water from their diet. The degu (Octodon degus), found in Northern Chile, lives in semi-arid desert country, known as matorral, which is characterised by evergreen scrub plants. Degus survive on limited amounts of water obtained primarily from their food, which comprises scrub foliage, grass and seeds. There is seasonal variation in the water content of plants; in summer the plant foliage dries out and contains just 3–6 per cent water; in winter, foliage contains 70–80 per cent water. Bozinovic et al. (2003) studied the phenotypic flexibility of water flux rate in Octodon degus. Water intake and efflux were measured by use of the doubly-labelled water technique in degus kept in a secure enclosure within the matorral. Urine osmolality was measured in wild-captured degus using microhaematocrit capillary tubes to obtain samples from the urethra. (Note: whereas osmolarity measures the number of osmotically active particles of a particular substance in a volume of fluid, osmolality measures the equivalent number in a mass weight of fluid. For most biological systems the molarity and molality of a solution are nearly exactly equal. For our purposes osmolarity and osmolality can be regarded as equivalent.)
| Measurement | Winter (June–August) | Summer (Dec–March) |
|---|---|---|
| Mean rainfall/mm | 245 | 12 |
| Body mass/g | 119.7 | 124.8 |
| Water intake/ml day−1 | 40.4 ± 91* | 10.3 ± 2.3 |
| Urine osmolality/mOsmol kg−1 | 1123 ± 472* | 3137 ± 472 |
Activity 8
Drawing on the data provided in Table 4, summarise the physiological strategy for water economy in the degu.
Answer
In winter when water content of plants is 70–80 per cent, the rate of water intake is relatively high at 40.4 ml day−1. Urine osmolality is correspondingly low at 1123 mOsmol kg−1. In contrast, in the summer the rate of water intake is relatively low at 10.3 ml day−1 and the degus produce a more concentrated urine, with an osmolality at 3137 mOsmol kg−1. The kidney is able to concentrate urine, thereby reducing water loss in the summer when the diet provides very little water.
Bozinovic et al. interpret the ability of the kidney of the degu to concentrate urine to 3137 mOsmol kg−1 as an example of phenotypic flexibility in the degu, in response to a lack of water during the summer. Variation in the osmolality of urine is not in itself unusual. After drinking a large volume of water, humans produce a dilute urine; the average osmolality in water-loaded volunteers has been measured at 101 mOsmol kg−1. Following 20 hours of dehydration, urine osmolality in the volunteers increased to 1004 mOsmol kg−1. Such responses result from the physiological regulation of body water content. Recall that the permeability of the epithelium of cortical and medullary collecting ducts is controlled by the hormone ADH (antidiuretic hormone, also known as vasopressin). (Figure 39 in Section 3.4 shows the feedback control of secretion of ADH, which results in the regulation of body fluid volume.)
