Week 3: Animals and ecosystems at the extremes
Use 'Print preview' to check the number of pages and printer settings.
Print functionality varies between browsers.
Printable page generated Saturday, 20 April 2024, 3:49 PM
Week 3: Animals and ecosystems at the extremes
Introduction

Two contrasting photographs, one of tall rock formations in a sandy desert and the other of penguins approaching the edge of an ice shelf in Antarctica.
Studying ecosystems in the most inhospitable places reveals a range of adaptations to survival. Desert or polar ecosystems seem remote, but their links with other ecosystems are very important.
Conditions in deserts and around the poles are harsh and the organisms that live in these habitats have a range of adaptations that enable them to live there, though often on the margins of survival. So, understanding how organisms survive is part of our understanding of the ecosystems as a whole. Studying how the organisms fit into an ecosystem involves considering a number of features. You will look at the integration of behaviour anatomy, physiology and biochemistry in diverse vertebrates that live in extreme conditions.
1 Deserts

The Wahiba Sands Desert, Oman, Middle East. There are occasional small trees and a few scrub plants.
Deserts have a unique climate, with characteristic organisms. In such an extreme environment, organisms will develop their own ‘niches’. A niche encompasses the role of an organism in a particular ecosystem, the habitat, how it eats, what it eats, and its predators.
There may be empty niches in a habitat. An invader may take over a niche by ejecting the species currently occupying it. In general, two species cannot occupy the same niche in the same geographical location.
In desert ecosystems, insectivorous, herbivorous and seed-eating niches are occupied by small animals, including arthropods, lizards, small birds, rodents, squirrels and shrews. Medium and large-sized animals such as hares, gazelle, camels and ostrich occupy grazing and browsing niches. Predators include foxes, e.g. kit fox (Vulpes macrotis) and cats, e.g. cougar (Puma concolor) in the deserts of the southern USA and Mexico, and Rüppell’s fox (Vulpes rueppelli) in the Arabian desert. Desert vertebrates make use of a variety of microenvironments and their associated microclimates, small-scale areas in which the climate is different from that of the habitat as a whole.
For example, in a desert ecosystem, a cavity beneath a rock, a microenvironment, would have a lower ambient temperature (Ta) than the surface and hence a different microclimate. A hyper-arid sandy desert, such as the Arabian desert, has a relatively low variety of microenvironments and associated microclimates available for vertebrates. Nevertheless, the sand at a few centimetres depth is significantly cooler than at the surface, and provides a relatively cool microenvironment for animals. In contrast, American deserts such as the Sonoran have a diverse range of microenvironments, and contain a richer diversity of vertebrate species.
Although our discussion here is restricted to vertebrates, you should be aware that many invertebrates, particularly insects, inhabit desert environments, and they provide an important food supply for many desert birds and mammals.
1.1 On size and shape
The ways in which animals interact with the environment is affected by their body size and shape.
One way to classify desert animals is in terms of the range of body sizes and the rate of evaporation.
The logic of this classification can be appreciated by the following exercise. If you represent a small animal by a cube, and then make a larger scale model of it twice natural size, the linear dimensions of the larger animal would all be twice as large (Figure 4).
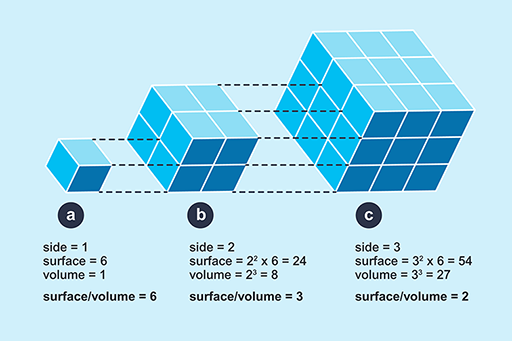
This is an illustration showing three cubes. There are no units of measurement: Cube A is a 1 by 1 by 1 cube; Cube B is a 2 by 2 by 2 cube; and Cube C is a 3 by 3 by 3 cube. Underneath Cube A is written ‘side = 1’, ‘surface = 6’, ‘volume = 1’ and ‘surface/volume = 6’. Underneath Cube B is written ‘side = 2’, ‘surface = 2 squared times 6 = 24’, ‘volume = 2 cubed = 8’ and ‘surface/volume = 3’. Underneath Cube C is written ‘side = 3’, ‘surface = 3 squared times 6 = 54’, ‘volume = 3 cubed = 27’ and ‘surface/volume = 2’. This illustration shows a cube with sides of 1 unit length, next to cubes with sides of 2 and 3 unit lengths. Underneath each cube are measurements of ‘side’, ‘surface’, ‘volume’ and ‘surface/volume’.
However, the surface area of the model would not be increased by a factor of 2, nor would the volume, as can be seen by comparing Figure 4a and 4b. If the linear dimensions double; the surface area increases by a factor of 4 (22) and the volume by a factor of 8 (23). So the ratio of surface area to volume is lower in a large animal than a smaller one. Since heat is transferred at the surface, a small animal has greater potential for rapidly gaining and losing heat than a larger one because of its relatively large surface area. A smaller animal also has greater relative potential for evaporative water loss through its large area of skin, relative to its volume.
However, animals are not cube-shaped, and certain desert species have features that can increase their surface area relative to their volume.
Activity 1
Consider this question and note your answers in the box below
What desert animals that you know of from your general knowledge have features that increase their surface area relative to their volume?
Answer
An obvious adaptation that increases surface are is large ears and so you might have chosen the desert fox, jerboa, the jack rabbit or the elephant. A more unusual adaptation is found in the frilled neck lizard, which has a flap of skin that it can extend both for display and to regulate body temperature.
1.2 Behavioural strategies of evaders

Cyclorana platycephala, is known as the water-holding frog and Neobatrachus sudelli is the painted burrowing frog. Both spend much of their lives in burrows, evading the hot, dry conditions in the Australian desert in summer.
Small animals, classified as evaders, include desert amphibians and reptiles, and also mammals – rodents and insectivores. The term ‘evaders’ refers to the animals’ behaviour, which helps to prevent overheating of the body on hot sunny days, and avoids the need for cooling by evaporative water loss, which is not feasible for small animals living in an arid habitat. Evaders make use of microenvironments such as shady rock crevices, underground burrows and shade cast by plants, for behavioural thermoregulation. Evaders also prevent excessive cooling of the body by behaviour, retreating to shelter when the ambient temperature (Ta) plummets at night.
The ultimate evaders are desert frogs such as Cyclorana spp. and Neobatrachus from Australia, which spend most of the year in aestivation, inside a burrow. Aestivation is a special kind of dormancy, which enables animals to survive lack of water and high Ta during a hot dry season. During the short rainy season, desert frogs accumulate water in the bladder, where it remains during aestivation. A famous example, Cyclorana platycephala, is known as the water-holding frog; aboriginal people used to dig up the aestivating frogs and squeeze them, in order to collect and drink the water.
During aestivation, the frogs are protected from losing water to the dry soil in the burrow by a cocoon. At the end of the rainy season, the frogs burrow into the soil, and the skin undergoes a type of moulting process in which layers of epidermis are separated from the body but not shed, forming a protective cocoon, covering all parts of the body apart from the nostril openings. The cocoon thickens, becoming heavily keratinised, and prevents loss of water from the frog’s body during the 9–10 months of aestivation. At the start of the rainy season, heavy rain with consequent seepage of water into the frogs’ burrows, stimulates the frogs to emerge. Breeding and feeding occur during the short wet season.
1.3 Desert reptiles
Reptiles with a scaly keratinised skin are not so prone to evaporative water loss as amphibians and are the vertebrates that you are most likely to see on a visit to a desert. Reptiles are ectotherms and rely on solar radiation for warming the body and maintaining high body temperature (Tb) during the day. Desert reptiles have no problem in gaining heat for maintaining Tb at a high level on hot sunny days (Figure 7).
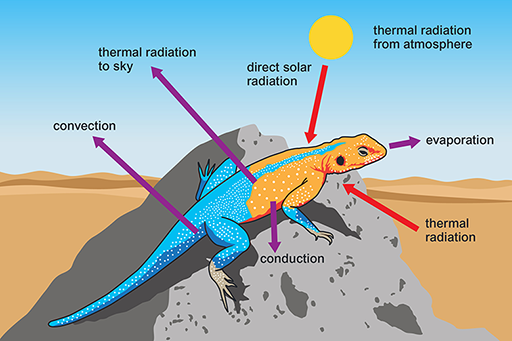
This is an illustration of a lizard on a rock. There are arrows pointing away from the lizard which read ‘convection’, ‘thermal radiation to sky’, ‘evaporation’ and ‘conduction’. There are also arrows pointing towards the lizard which read ‘direct solar radiation’ and ‘thermal radiation’. There is also a label which reads ‘thermal radiation from atmosphere’. This illustration of a lizard on a rock has arrows pointing away from the lizard which read ‘convection’, ‘thermal radiation to sky’, ‘evaporation’ and ‘conduction’. Arrows pointing towards the lizard read ‘direct solar radiation’ and ‘thermal radiation’.
What are the sources of energy gain and routes of heat loss for the lizard?
The lizard gains heat energy via thermal radiation from the Sun, the atmosphere and the ground. Heat energy is lost via conduction from the body to the ground, by evaporative water loss, convection and thermal radiation to the sky. On a hot sunny day, more heat is gained than lost, and it is important for a desert reptile to avoid overheating. It is equally important to reduce loss of body heat when Ta plummets at night or during the winter.
During the day, reptiles may move between warm and cool areas in order to maintain Tb. This movement between warm and cool areas for maintaining body temperature is called shuttling. Those species that maintain high stable Tb when environmental conditions allow by adopting heliothermic (sunbasking) strategies, are called thermal specialists. In contrast, there are some species, known as thermal generalists, which allow their Tb to fluctuate and decline, even when they could shuttle between sun and shade to maintain high stable Tb during the day, or use their burrow at night to prevent cooling of Tb to the outside Ta. Bedriagai’s skink (Chalcides bedriagai) is a thermal generalist, preferring to spend a lot of time hiding under rocks rather than basking in the Sun.
The side-blotched lizard (Uta stansburiana), found in the Sonoran desert, is a typical thermal specialist. It is a small species, only 4–6 cm long when full grown.
In the morning, Uta warms by basking, initially orientating itself at right angles to the Sun’s rays and flattening the body against the substratum for maximum exposure to solar radiation. When warmed Uta turns the body so that it faces the Sun while resting. Uta maintains Tb around 36–38°C. Active foraging for insects, scorpions and spiders may overheat the body, and for cooling off, especially around noon, Uta moves to the shade of rocks and scrubby bushes. Shuttling in this way enables this species to stay active during the day for most of the year except in areas where winter temperatures dip to freezing.

The Moorish gecko (Tarentola mauretanica) is active at night, for a few hours after sunset. In the morning it can be found basking in the sun.
A few desert reptiles are nocturnal; the Moorish gecko (Tarentola mauretanica), is found in arid regions in North Africa (also in Spain, France and Greece, so it is not restricted to deserts).
Tarentola is most active for a few hours after sunset. During the night, it’s Tb is as low as 18°C, and can fluctuate by up to 11°C. Those lizards that tolerate wide fluctuations in Tb, even when they could use features of the environment to maintain a steady Tb, are known as thermal generalists. The Moorish gecko is a thermal generalist at night, when it is active rather than resting in its burrow. During the early morning the Moorish gecko basks in the sunlight.
The advantage to the gecko of warming up in the morning is uncertain, but it is possible that a physiological process such as digestion of the food eaten during the night requires a higher Tb than the gecko can maintain at night.

The Mojave fringe-toed lizard (Uma scoparia) is a ‘sand-swimmer’ and its dorsoventrally flattened body and shovel-shaped head facilitate movement through the sand.
Sheltering in the available shade in the desert, or being active at night, are simple strategies for keeping Tb below lethal levels. In sandy desert areas, the sand itself plays an important role in behavioural thermoregulatory strategies. The Mojave fringe-toed lizard (Uma scoparia) is restricted to fine, wind-blown sand, e.g. in dunes, dry lake beds and desert scrub in the Mojave desert. Burrows in sand collapse immediately or soon after the animal has moved on, so animals buried in sand rely on air trapped between sand particles for breathing. Uma is a ‘sand-swimmer’ and its dorsoventrally flattened body and shovel-shaped head facilitate movement through the sand, which is especially important when escaping from predators such as snakes.
The eyelids are protected from sand by large eyelid fringe scales. The digits have large lamellar fringes, elongated scales, especially long on the hind feet, which enable the lizards to run at speed on the sand surface. Uma grows up to about 110 mm in length, and its activity pattern is diurnal, varying according to ambient temperature. In March and April Uma is active for short periods because of the low spring temperatures in the Mojave. In summer, from May to September, the lizards are active during mornings and late afternoons, feeding on insects and plants. Sand-swimming lizards are also found in the Namib desert and include the wedge-snouted sand lizard (Meroles cuneirostris).
Desert burrows
Look at the way in which the surface temperature of sand can change through a day (Figures 10 and 11).
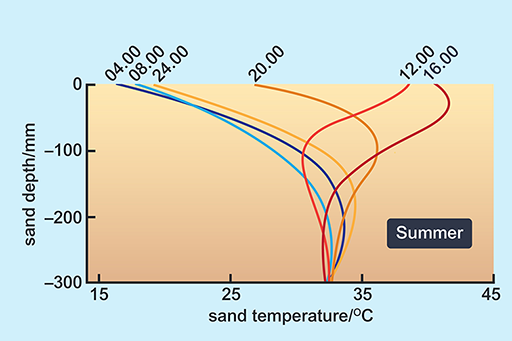
This graph shows the temperatures (taken at 6 times a day: 4am, 8am, 12pm, 4pm, 8pm and 12am) below the sand surface on a dune in the Namib desert on a summer day. The X axis is labelled ‘sand temperature/degrees C’ ranging from 15 degrees to 45 degrees. The Y axis is labelled ‘sand depth/mm’ ranging from -300 mm to 0 mm. At -300 mm, all 6 times of day have sand temperatures of 32 degrees. On the surface however, at 4am the temperature is 16 degrees, at 8am the temperature is 17 degrees, at 12pm the temperature is 38 degrees, at 4pm the temperature is 40 degrees, at 8pm the temperature is 27 degrees and at 12am the temperature is 18 degrees. This graph shows the temperatures (taken at 6 times a day: 4am, 8am, 12pm, 4pm, 8pm and 12am) below the sand surface on a dune in the Namib desert on a summer day. The temperatures range from 15 to 45 degrees Celsius.
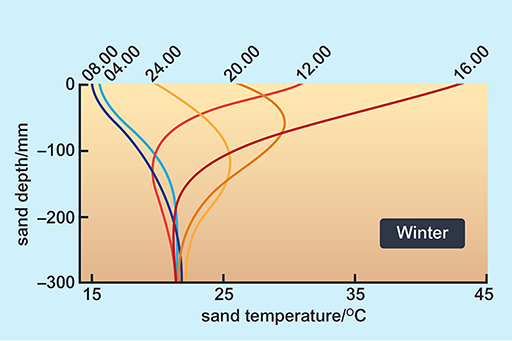
This graph shows the temperatures (taken at 6 times a day: 4am, 8am, 12pm, 4pm, 8pm and 12am) below the sand surface on a dune in the Namib desert on a winter day. The X axis is labelled ‘sand temperature/degrees C’ ranging from 15 degrees to 45 degrees. The Y axis is labelled ‘sand depth/mm’ ranging from -300 mm to 0 mm. At -300 mm, all 6 times of day have sand temperatures of 22 degrees. On the surface however, at 4am the temperature is 16 degrees, at 8am the temperature is 15 degrees, at 12pm the temperature is 31 degrees, at 4pm the temperature is 43 degrees, at 8pm the temperature is 26 degrees and at 12am the temperature is 18 degrees.
Although the temperatures of sand at various depths in the Mojave desert would not be precisely the same as those in the Namib, the physical characteristics and thermal environment provided by dry sand are broadly the same in all deserts at similar Ta.
A benign temperature is available below the surface at all times of the day in both seasons, in spite of extremes on the surface. These surface temperature extremes are not very different in summer and winter. The high afternoon surface temperature in winter is due to hot, dry winds (Berg winds) that reach the desert in the winter months.
Burrowers
Burrows provide important microenvironments for many desert evaders, and their structure and use vary between species. The desert tortoise (Xerobates agassizii) lives in deserts in the USA and Mexico, and feeds on annual herbs, cacti and shrubs, obtaining most of its water from the plants.

Another example of an animal that evades extreme environmental conditions, the desert tortoise (Xerobates agassizii).
In the Mojave desert, the tortoises live in sandy areas as well as rocky hillsides, including scrub-type vegetation, Joshua tree/yucca and creosote bush/ocatillo habitats. For the tortoises, burrows are important refuges for thermoregulation, summer aestivation and winter hibernation. Tortoise burrows in the Mojave desert are extensive and can be up to 12 m long; the same burrows are used for many generations, and are shared with other species such as burrowing owls and ground squirrels. Each desert tortoise may use up to 12 burrows in its home range and each burrow is used by different tortoises at different times. For short rest periods during the day tortoises dig shallow depressions, known as pallets, which barely cover the carapace.
Bear in mind that when occupied by a tortoise, a burrow’s relative humidity may rise to 40 per cent because of the tortoise’s water loss by evaporation from the lungs, exposed skin and eyes. Stable Ta and humidity in the burrow protect the tortoise from extremes of high Ta and from winter frosts. It was noticed that tortoises are fussy about the burrow selected for resting. At the end of foraging, tortoises were observed to enter and leave several burrows before settling. Mojave desert tortoises are active between March and June, a time when the winter rains have stimulated the growth of annual plants, providing abundant food for the tortoises. The tortoises begin foraging during the morning but usually by noon they have moved into pallets and burrows to shelter from high Ta. At night, burrows provide shelter from low Ta and also protection from nocturnal predators such as kit foxes and badgers. By the end of June, when surface temperature may reach 60°C, and annual plants have dried up, the tortoises retreat to their deep burrows and aestivate, a behaviour that helps to conserve body water. During aestivation, up to a quarter of the tortoises’ body mass may be water stored in the bladder. Occasionally an aestivating tortoise emerges to drink during summer thunderstorms. In the eastern Mojave desert tortoises are active for most of the summer because there, summer rainstorms provide sufficient new plant growth. For their winter hibernation, tortoises aggregate in the burrows; up to 25 individuals have been found in one burrow. Hibernation lasts from October to the end of February, and during this time Tb of the tortoises is the same temperature as the burrow, around 5–16°C in winter. Note therefore that hibernation in the desert tortoise is not the same physiological process as it is in hibernating mammals . Reptiles do not regulate Tb physiologically during hibernation; Tb is the same as burrow Ta. You will find that in some references, reptile ‘hibernation’ is termed ‘brumation’.
Mammalian desert burrowers
You may be surprised to learn that like desert ectotherms, small desert rodents also depend on burrows for thermoregulation. Merriam’s kangaroo rat (Dipodomys merriami) is a typical evader, living in the Sonoran desert, Arizona, and in Death Valley, California, two of the hottest and driest areas in the Western Hemisphere.

Merriam's kangaroo rat (Dipodomys merriami) is a desert mammal that spends most of the day in a burrow, emerging at night when it is cooler.
Individuals live in a maze of burrows, which they defend. They remain in their burrows during the day, and often plug the entrance with soil. At night kangaroo rats emerge from their burrows for just two hours to collect seeds, in particular seeds of the creosote bush, which they push into their cheek pouches, returning at intervals to empty the food into their burrow. In this way, food caches are built up; kangaroo rats always eat inside the burrow, drawing on their food cache. Inside the burrow, the air is cooler and more humid than above the ground, as moisture from respiratory water loss accumulates. Measurements made on similar burrows in the Negev desert, Israel, showed Ta of around 26°C at 1 metre depth for 24 hours per day when ambient temperature above ground ranged from 16–44°C. However, not all small desert animals can burrow.
The desert wood rat (Neotoma lepida) lives in deserts in the southern USA, including Death Valley, California. Wood rats do not burrow but build elaborate houses around the base of cacti or shrubs, amongst a patch of agaves, or beneath a rock outcrop. Wood rat houses can reach huge sizes and their interior is significantly cooler, by about 5°C, than the outside during the heat of the day. Desert wood rats shelter in their houses during the day, and emerge to forage at night, eating creosote bush, cholla, prickly pear cactus and agave.
1.4 Behavioural strategies of evaporators
Evaporators are animals that depend on sufficient water intake to enable them to cool Tb by evaporation. Few of these species can survive in deserts, and those that do either live on the edges of deserts where they can access water, or have behavioural and physiological adaptations that reduce reliance on evaporative cooling. So for evaporators, evasion may be an important part of their thermoregulatory strategy. Evaporators include medium-sized mammals such as jack rabbits, dogs, foxes, and also desert birds such as larks.
The jack rabbit (Lepus californicus) is a hare, living in the Sonoran and Mojave deserts. Jack rabbits do not burrow, although they are quite small, weighing about 2 kg.
A jack rabbit would need to lose at least four per cent of its body mass per hour to thermoregulate by evaporation. There is little or no free water around; water is obtained from the diet, green plants, including cacti in the summer. The classic work of Knut Schmidt-Nielsen (1965) showed that behaviour is important for the jack rabbit’s survival. During the hottest part of the day the animal chooses a shaded depression in the ground, often in the lee of a bush, in which it crouches (Figure 6).
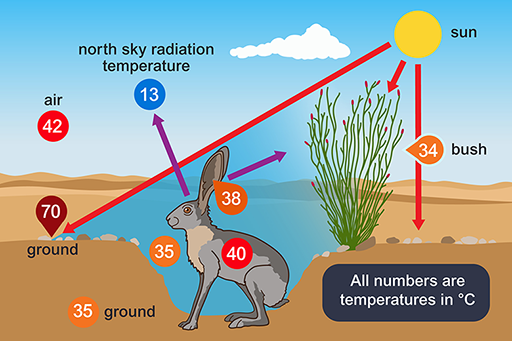
This illustration shows a desert jack rabbit in a shaded depression behind a bush. Arrows are pointing from the sun at the ground and the bush. Arrows are also pointing away from the rabbit into the sky and towards the bush. Different elements are labelled with their temperature in degrees Celsius. The bush is labelled as 34, the rabbit’s body at 40, the rabbit’s ears at 38, the air just in front of the rabbit at 35, the air as 42, the ground where the sun is shining as 70 and the ground below the surface as 35. There is also a label which reads ‘north sky radiation temperature’ which has the number 13 underneath it. This illustration shows a desert jack rabbit in a shaded depression behind a bush, with numbers representing temperature. Areas in direct sunlight are hotter temperatures than those in the depression.
The bottom of such a depression has a much lower temperature than that of the rest of the surface, the hot desert wind and much of the radiation passing over the animal’s head. From its sheltered position, the jack rabbit’s large radiator-like ears can be exposed, not directly to the Sun, but to a clear blue sky. The radiation temperature of the north sky at midday is only 13°C so if the ears, which are richly vascularised, have a temperature of 38 °C, and have a surface area of 400 cm2, are directed towards the sky, they can radiate about 13 kJ h−1, which is about half of the animal’s metabolic heat production. The jack rabbit forages during the night.
The kit fox (Vulpes macrotis) lives in the Sonoran, Mojave and Great Basin deserts in southwestern USA. Kit foxes have very large ears, which are thought to provide an increased surface area for cooling the body.
They are carnivores, and hunt at night, preying on kangaroo rats, tortoises and jack rabbits, and occasionally catching ground-nesting birds, reptiles and insects. They reduce evaporative water loss by spending the day in underground dens, emerging at sunset to begin hunting. The physiological importance of dens for desert foxes should not be underestimated. By remaining in the den during the day, a desert fox reduces drastically the need for panting, a mechanism used by foxes and dogs for cooling the body by evaporative water loss.
A few species of small birds live in the most extreme deserts. Dune larks (Mirafra erythroclamys) are the only birds that live year round in the Namib sand sea, one of the driest regions of the world. Dune larks feed on insects and spiders, which they collect during the day, while walking over the sand surface; they also peck insects from just below the sand surface. In winter the birds feed on seeds blown in from adjacent grass land. The scarcity of water in the Namib sand sea means that dune larks drink rarely and the birds rely on water in their food and on metabolic water. Birds do not sweat, but they use both cutaneous and respiratory evaporative water loss for cooling the body. During the hottest part of the day, from around 12.00 to 15.00, dune larks seek shade and stand still. Presumably this behaviour helps the birds to cool Tb and reduces evaporative water loss.
Taxidermic mounts have been used to determine operative environmental temperature, Te, for the birds. Te is the temperature that an animal would reach in the environment if it was biologically inactive, i.e. only the physical characteristics of the animal are taken into account. It is defined, in physical terms, as the temperature of a black body of uniform temperature, in an identical situation to that which the animal occupies, with the same values for conduction, convection and radiation. As the definition is purely physical, it is possible to make models of animals and to use them to measure Te experimentally.
The results of these experiments suggest that in winter, the strategy of finding a shady spot during the hottest part of the day lowers Tb sufficiently, so there is no need for physiological cooling, in particular evaporative water loss, for maintaining Tb
While desert animals classed as ‘evaporators’ could use evaporative cooling for maintaining Tb at high Ta, the need for this is avoided by simple behavioural strategies. Nocturnal foraging and daytime use of dens, burrows and shade for cooling reduce the need for physiological cooling by evaporative water loss, thereby conserving water.
1.5 Behavioural strategies of endurers

The Dorcas gazelle (Gazella dorcas) is the most desert-adapted of all gazelles and can survive without drinking for long periods, relying on water derived from it's food.
Endurers are defined as large desert mammals such as oryx and camel, and large desert birds, including ostrich and emu. The term ‘endurers’ suggests that these animals are forced to endure the extreme conditions of the desert climate because they cannot shelter from high Ta and intense solar radiation during the day or low Ta at night, as they are too large to hide in burrows or dens. Nevertheless, in spite of their size, endurers do take advantage of aspects of the environment for cooling by means of behavioural strategies.
Large mammals tend to be inactive during the hottest part of the day, thereby reducing metabolic heat production. The Arabian oryx (Oryx leucoryx) lives in the Arabian desert, including areas where free-standing water is rarely if ever available. On hot days oryx dig into the sand with their hooves, exposing the cool sand below the surface, and sit in the depressions. Body heat is lost to the cooler sand by conduction. Where possible, the oryx also spends time sitting in the shade of evergreen trees (Maerua crassifolia) during the hottest part of the day. Oryx forage at night during the summer, avoiding exposure to high Ta and intense solar radiation. They feed on grasses and rely on the water content of the plants for their intake of water.
Dorcas gazelle (Gazella dorcas) live at the borders of the Sahara desert and are the smallest species of gazelle, weighing just 15–20 kg. They have very long limbs in proportion to their body size, and large ears: both features maximise any convective cooling caused by breezes. Dorcas are described as the most desert-adapted of all gazelles, as like the oryx, they are reputed to be able to survive without drinking any water at all. Their feet are splayed, an adaptation for walking and running on sand. Dorcas gazelle graze and browse at night and at dawn and dusk, feeding on leaves, flowers and pods of acacia trees, and using their hooves to dig for bulbs.
Long limbs, tails or necks provide large surface areas from which heat can be dissipated, and behaviour patterns may maximise loss of heat from these areas. The ostrich (Struthio camelus) is the largest living bird, weighing up to 150 kg. Ostriches forage during the day. The birds select plants with high water content when grazing, especially during times of water shortage. The naked neck of the ostrich and its long naked legs provide a large surface area for convective and radiative cooling, especially in breezy conditions. The ostrich uses behaviour to enhance the cooling effects of feather erection at a high ambient temperature and incident solar radiation. Sparsely distributed long feathers on the dorsal surface of the bird erect in response to warming of the skin, thereby increasing the thickness of the insulation between solar radiation and skin. The gaps between the feathers allow through air movements, which cool the skin by convection. The birds supplement the physiological response during the hottest part of the day by orientating themselves towards the Sun and bowing out their wings away from the thorax, forming an ‘umbrella’ which shades the exposed thorax. The naked skin of the thorax acts as a surface for heat loss by both radiation and convection. At night when ambient temperatures plummet, ostriches conserve heat by folding the wings close to the thorax and tucking the naked legs under the body while they sit on the ground. The dorsal feathers respond to low Ta by flattening and interlocking, which traps an insulating layer of air next to the skin, and keeps most of the skin at 34.5° C.
Evaporative water loss is the most effective means of reducing body temperature during heat stress. However, in deserts, very little, if any, free-standing water is available. For all groups of desert vertebrates, behavioural strategies for maintaining Tb play a crucial role in preventing overheating of the body, which reduces the need for evaporative cooling and thereby conserves water. In the following section, we will see how in desert vertebrates, behavioural strategies for controlling body temperature are integrated closely with biochemical and physiological mechanisms.
Desert animals are classified in terms of their body size and physiology into three groups: evaders, evaporators and endurers. The logic for this classification is that the smaller the animal, the larger its surface area to volume ratio. Small animals therefore gain and lose heat faster than large animals, warming rapidly when exposed to intense solar radiation, and cooling rapidly at night.
Small endothermic evaders, e.g. kangaroo rats, rest in cool microenvironments, e.g. shade or burrows, during the day. Lizards, ectothermic evaders, regulate Tb during the day by shuttling between sun and shelter. They avoid night-time hypothermia by resting in burrows. Nocturnal evaporators, e.g. kit foxes, remain in cool dens during the day. Some endurers, large species such as the oryx, graze nocturnally in summer, sitting in shade during the day. Behavioural strategies for avoiding intense solar radiation link intimately to physiology. Such behaviour prevents large fluctuations in Tb and conserves water by removing the need for evaporative cooling, which is of crucial importance in deserts where water is scarce.
1.6 Camels and humans as desert dwellers
Both humans and camels live in desert conditions and both rely on evaporative cooling to regulate their body temperatures. However, as this classic Open University video makes clear, the camel handles its water balance better than humans do, as well as having other adaptations that help it survive in a desert ecosystem.

Transcript
Camels and humans as desert dwellers
[MUSIC PLAYING]
[SINGING]
[WATER RUNNING]
[SPEAKING A FOREIGN LANGUAGE]
2 Cold environments

Saxifraga oppositifolia, a plant of the Arctic, and mountainous regions further south. Once the snow melts it flowers throughout the spring and summer, producing small pink flowers.
The Arctic regions are the exact opposite of deserts as far as severe climate is concerned. Organisms in the Arctic regions have adapted to habitats influenced by extreme cold, resulting in short growing seasons for plants, land that produces little food, and lack of shelter. Some animals migrate and thus avoid the extreme cold for large parts of the year.
At high latitudes, the Sun’s rays always strike the Earth at a large angle from the vertical so they travel through a thicker layer of atmosphere and are attenuated by the time they reach the ground. Because the Earth’s axis of rotation is inclined to its path around the Sun, there are large seasonal changes in day length and the Sun is continuously below the horizon for a period in winter and continuously above the horizon for an equivalent period in summer. The range of annual temperature change is much greater at higher latitudes, and in mid-winter (January and February), the range about the mean is more than 12 °C. In polar climates, the temperature can change abruptly and often unpredictably. In coastal areas, the sea keeps the climate much more equable. Further inland, fluctuations in temperature are even greater. Polar organisms are thus adapted both to the extreme cold and to abrupt fluctuations in temperature.
The Arctic Circle (66° 30′N), and the equivalent latitude in the Southern Hemisphere, are defined as the latitude above which the Sun is continuously below the horizon for at least one day each year. Warm, moist air from the temperate zone rarely reaches high latitudes, so in most polar areas precipitation is low. Much of the water is locked away as ice, which has a low vapour pressure, and the air is very dry (often as dry as a tropical desert) and ground water is inaccessible to plants as well as to animals.
Terrestrial environments in the Arctic are, by geological standards, relatively new, most of the land having been completely covered with a thick layer of ice as recently as 10 000 years ago. Consequently, the soil is thin and fragile, and poor in organic nutrients. The optimum temperatures for plant growth do not coincide exactly with peak sunshine. At Longyearbyen, continuous daylight begins in late April, but the mean temperature does not rise above 0 °C (and so the snow and ice do not melt) for another two months.
These circumstances, combined with the severe climate, mean that the growing season for plants is short but intensive, and total productivity on land is low, producing little food and still less shelter for animals.
2.1 Life on land at high latitudes
Relatively few species of terrestrial organisms live permanently at high latitudes. For example, although the land area of Svalbard is about 62 000 km2, almost half that of England, there are only a few hundred species of insects and other invertebrates, two resident terrestrial mammals, the arctic fox (Figure 17a) and reindeer (Figure 17b), one bird (an endemic species of ptarmigan) and no reptiles, amphibians or completely freshwater fish.

Two animal species that are resident in the high Arctic latitudes, the arctic fox (Alopex lagopus) and a subspecies of reindeer Rangifer tarandus platyrhynchus.
However, many other species spend part of the year on or near the land, often while breeding or moulting: seasonal visitors include more than 30 species of migratory birds (various kinds of geese, auks, puffins, skuas, terns, gulls, and eider ducks and snow buntings), and mammals that feed in the sea, such as polar bears, walruses and several species of seal. The simple ecosystem on land and the severe, erratic climate tend to produce ‘cycles’ of population abundance followed by mass mortality or migration (e.g. lemmings in Scandinavia and Russia). Interesting physiological and behavioural adaptations to these fluctuations in food supply have evolved in some of the larger animals. The vast continent of Antarctica has no indigenous terrestrial vertebrates, although many birds, including penguins, skuas, terns and gulls, and six species of seal spend time on or near land.
Only two species of terrestrial mammal occur naturally throughout the year on Svalbard (although a few others have been introduced by humans during the past century). Figure 17a shows the arctic fox (Alopex lagopus), which also occurs throughout the Arctic, and in mountains at lower latitudes. The picture in Figure 17a, taken in late autumn, shows an adult in its long, dense winter coat. The summer coat is usually greyish brown, often with white markings. Alopex is bred in captivity for its fur, which can vary in colour from grey to bluish in winter, and chocolate brown to fawn in summer, hence the common names, silver fox or blue fox. Figure 17b shows a subspecies of reindeer (Rangifer tarandus platyrhynchus) that is endemic to Svalbard. This picture was taken in July, when the vegetation is at its highest, and this young male is growing antlers for the mating season in September.
The situation in the polar seas is very different, which you will discover as you conclude your study of extreme ecosystems by learning about life in the polar seas.
2.2 Life in the polar seas
Life in the polar sea ice forms part of a web of interactions, which Dr Mark Brandon discusses with Brett Westwood as he considers the tiny life trapped in the sea ice that is the foundation for the entire food chain at the poles.
Transcript
Life in the polar seas
Seawater freezes at −1.9° C, but because of the anomalous relationship between the density and temperature of water, ice floats, insulating the water underneath from the cold air above. Except in very shallow areas, the sea-ice does not extend to the sea-bed, even at the North Pole. Storms and currents sometimes break up the ice, creating many temporary, and some permanent, areas of open water even at high latitudes in mid-winter. Such turbulence also oxygenates the water and admits more light, making the environment much more hospitable to larger organisms.
The movements of ocean currents are complex and may change erratically from year to year. This often results in an upwelling of deep water rich in nutrients and promotes high primary productivity in the sea. In most arctic regions, the sea is both warmer and more productive than the land. So at high latitudes there are many more organisms in the sea than on land, at least during the brief summer, and, as in the case of the baleen and sperm whales, some are very large.
Krill
You heard previously how Dr Mark Brandon and colleagues studied krill under the sea ice. In this video scientists from the British Antarctic Survey (BAS) are trawling for krill and sorting them for later analysis – some task!
As you watch listen out for answers to the following questions:
- What is the role of krill in the Antarctic food chains?
- How do the food chains in the polar seas compare with those introduced earlier by Professor David Streeter in the oak wood?

Transcript
Krill
DR. DAVID ROBINSON: This huge net is being used to trawl the polar seas for krill, as part of a research programme. In the folds at the bottom of the net are thousands of these prawn-like crustaceans. Krill are a key component of the main food web in the southern oceans.
MALE SPEAKER 1: Do you want me to--
MALE SPEAKER 2: In the net too.
MALE SPEAKER 1: Yeah.
MALE SPEAKER 2: I think there'll be more water on deck tomorrow morning.
DR. DAVID ROBINSON: They're an essential source of food for numerous animals, from fish and birds to the largest of the whales, the blue whale. In recent years, krill have even been harvested commercially.
FEMALE SPEAKER: Yeah.
MALE SPEAKER 3: Is there anything on that or is it just a surface layer of krill?
DR. DAVID ROBINSON: Sorting the sample is a painstaking task, as the scientists pick out individual krill to place them in trays for later analysis.
3 Apply your knowledge of ecosystems

A view of an ice berg and drift ice in Antarctic seas.
A number of interesting points came out of the previous section. Consider some other, wider questions about possible changes in the ecosystems and how they might affect life in the polar regions.
Here are some questions that might prompt your thoughts about change in polar ecosystems and food chains.
- What kinds of physiological adaptations to fluctuating food supply have organisms in the Arctic regions made?
- What kinds of behavioural adaptations do organisms in the Arctic regions have that specifically suit the ecosystems they are a part of?
- How do emperor penguins’ breeding patterns fit the harsh ecosystem they are a part of?
- How would leopard seals be affected if the penguin population experienced a boom or a decline? What would this mean for the rest of the ecosystem they are a part of?
4 Week 3 quiz
This test is about the physical characteristics of extreme ecosystems and the adaptations that some animals have to the harsh conditions.
Complete the Week 3 quiz now.
5 Review of Weeks 1 to 3

Photos of two different ecosystems in Northern Europe, a woodland and a grass meadow with wild flowers.
These three weeks have introduced you to the concept of an ecosystem and the debate about how to set the boundaries of such systems. Energy is the key link within ecosystems and the pathways by which energy flows can be explored within an ecosystem by examining the food chains that are present.
In some types of ecosystem the food chains are long, complex and many-branched. In the polar seas you have found that they can be very short, such as the 3-step chain from plankton, through krill to the largest animal on the planet, the blue whale.
Some of the examples of animals in ecosystems have introduced you to the concept of adaptation. You saw squirrels flying through the air and you may have appreciated links between their adaptations and those of other animals, such as the flying lizard and the flying snake. These adaptations to gliding enable faster travel through the complex, forest environment. Gliding shows how similar solutions can arise during evolution in unrelated groups of organisms.
In the second half of this course you will be looking at the impacts humans have on ecosystems, but major events occurring on the planet may also have impacts. Volcanic eruptions and tsunamis are obvious examples. This video shows a large scale event in an ice sheet in Antarctica. The effect on the area itself is substantial, but could it have wider implications? This is a larger question which you should ponder on and come back to when considering human impacts on ecosystems.

Transcript
Large scale change
A continuing theme has been that of conservation. How do you study ecosystems and then conserve them? Conservation raises a whole series of questions. For example, should conservation efforts be concentrated at the individual species level or should they all be directed to conserving habitats?
Take this question away with you as you finish the next three weeks of the course. You will appreciate that there is no simple answer, but if you know what you have in an ecosystem then at least you can start to decide what action to take to conserve it. Identifying animals, plants and fungi is, therefore, an essential part of the study of ecosystems. Next week, you will take part in an activity about identifying organisms in an ecosystem, using the iSpot website to post observations.
If you would like a short break or tofind out more about animals living at the extremes visit our Ecosystems area on OpenLearn.
You can now got to Week 4.
References
Acknowledgements
Except for third party materials and otherwise stated in the acknowledgements section, this content is made available under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Licence.
The material acknowledged below is Proprietary and used under licence (not subject to Creative Commons Licence). Grateful acknowledgement is made to the following sources for permission to reproduce material in this course:
Course image: courtesy of Dr. David J. Robinson, The Open University
Figure 1: Ron and Patty Thomas; and oversnap both iStockphoto.com
Figure 2: courtesy of Dr. David J. Robinson, The Open University
Figure 3: Mlenny; iStockphoto.com
Figure 5: ColbyJoe; iStockphoto.com
Figure 6: Robinson, M., A Field Guide to Frogs of Australia © New Holland Publishers
Figure 8: Dr Peter Davies
Figure 9: Brad Alexander
Figure 12: Tigerhawkvok; https://creativecommons.org/ licenses/ by-sa/ 3.0/
Figure 13: Dr Lloyd Glenn Ingles, CA Academy of Sciences
Figures 15, 18 and 19: courtesy of Dr. David J. Robinson, The Open University
Figure 16: zanskar; iStockphoto.com
Figure 17: (a) DmitryND; iStockphoto.com (b) Dr Angelika Renner; iStockphoto.com
Audio/Video
Krill in the Antarctic food chains: British Antarcti Survey: edited by The Open University, courtesy of British Antarctic Survey
Large scale event in an ice sheet in Antarctica; British Antarctic Survey
Desert Dwellers; courtesy of Getty Images
Every effort has been made to contact copyright owners. If any have been inadvertently overlooked, the publishers will be pleased to make the necessary arrangements at the first opportunity.
Don't miss out:
1. Join over 200,000 students, currently studying with The Open University – http://www.open.ac.uk/ choose/ ou/ open-content
2. Enjoyed this? Find out more about this topic or browse all our free course materials on OpenLearn – http://www.open.edu/ openlearn/
3. Outside the UK? We have students in over a hundred countries studying online qualifications – http://www.openuniversity.edu/ – including an MBA at our triple accredited Business School.
Copyright © 2019 The Open University

