3 Measurements on the microscope
Sometimes it is important to measure the size of a cell, the distance between cells or the number of cells in a given area. Most light microscopes have the option to add a graticule for determining distances or a grid into the light path, to allow such measurements to take place.

Transcript
These elements are usually added by exchanging one of the eyepieces for another with a graticule built into it. Therefore, the appearance of the graticule is fixed according to the eyepiece that is used; changing the objective does not change the appearance of the graticule.
A consequence is that the graticule has to be calibrated for each objective. This is most readily done by viewing a slide that has rulings etched into its surface, and observing how many units on the graticule correspond to, for example, 1 mm on the slide. Such a slide is shown below.
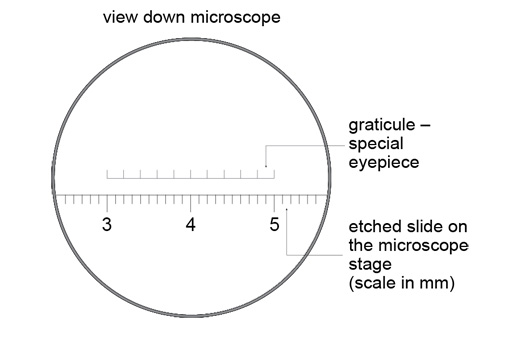
The video above shows you how to calibrate an objective using a graticule and etched slide on the virtual microscope, as well as introducing a grid feature that you can apply. The grid can be used to measure the number of items within a particular area, for example, the density of blood vessels or the numbers of cells within a defined area of tissue. These are skills you will use in later parts of the course.
