2.7 Another source of Al
Bauxite isn’t the only thing that goes into making an Al can. To find, mine, transport and purify bauxite into Al takes an enormous amount of energy.
The cost of the electricity used to split the bond between aluminium and oxygen in alumina makes up somewhere between one and two fifths (20–40%) of the total cost of aluminium. In fact, Al smelting plants use so much power that they are usually sited right next to power stations, in order to reduce the loss of power during the transmission of electricity over longer distances. There is, however, another way: recycling.
We already have a lot of pure aluminium hanging around – it’s in the coke cans, the car chassis and cooking foil that we use every day. Recycling this aluminium uses significantly less energy – around 5% of the energy required for mining, transporting and refining new aluminium.
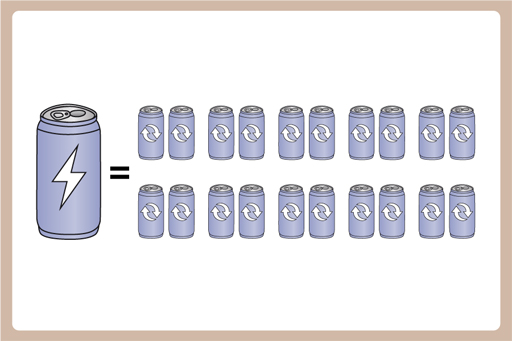
This means that with the energy it takes to make one new Al can, we could make 20 recycled ones.