2.3 Comparing ‘dry ice’ with sand
Earlier, you saw that carbon dioxide may be viewed as a molecular substance. The element below carbon in the periodic table is silicon.
And silicon also forms a compound with oxygen having a 1:2 ratio of the component atoms in its formula.
This is silicon dioxide – SiO2 – also known as silica, and is the main component of sand, but at this point the key thing to note is that it has the same ratio of atoms in its empirical formula as carbon dioxide.
But here’s the difference.
In solid carbon dioxide, two of the oxygen atoms around each carbon were much closer than others, so a CO2 molecule may be clearly identified.
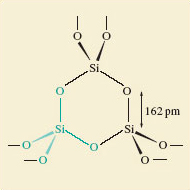
Now consider silica (Figure 6).
-
What atoms, and how many atoms surround each silicon atom in SiO2?
-
Silicon is surrounded by four oxygen atoms.
A better description would be to say; each silicon atom sits at the centre of a tetrahedron of oxygen atoms: and as shown in Figure 6 they are all at the same distance (162pm).
There is no evidence of discrete SiO2 molecules.