5 Cataloguing chemical substances
At this point in the course a useful classification systems for chemical substances has emerged.
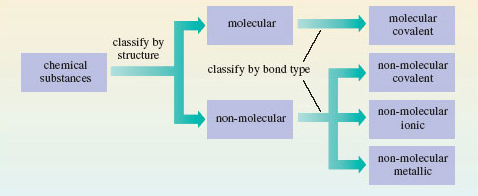
As you’ve seen they may be divided into molecular and non-molecular types, largely on the basis of their structures. Then a further division can be made according to the major source of the chemical bonding holding their atoms together. In molecular substances, the bonding is covalent, but in the non-molecular class, it may be covalent, ionic or metallic.
This classification is summarised in Figure 8.
Consider the compounds IBr, CaCl2 and CaMg2. One is ionic, one is covalent, and one is metallic. Identify which is which, and match each compound to one of the descriptions below. In each case, suggest whether the compound is molecular or non-molecular.
- i.White solid that melts at 782°C. It is a poor conductor of electricity in the solid state, but a good one when melted or dissolved in water.
- ii.Brown-black solid that melts at 41°C to give a liquid with low electrical conductivity.
- iii.Silvery-looking solid that melts at 720°C. Whether solid or molten, it is an excellent conductor of electricity.
CaCl2; (ii) IBr; (iii) CaMg2.
The properties listed are characteristic of (i) an ionic substance, (ii) a molecular covalent substance, and (iii) a metallic substance. CaCl2 is a combination of elements from the extreme left and extreme right of the periodic table, so the electronegativity difference will be large and CaCl2 will be the ionic compound; such compounds are non-molecular. IBr will be covalent because it is a combination of elements of high electronegativity from the extreme right of the periodic table. CaMg2 will be a metallic alloy because it is a combination of metallic elements with low electronegativity, Such alloys are non-molecular.
So, up to this point you’ve met some elementary ideas about chemical bonding, you’ll be developing these further in the next session and find out more about how the type of bonding affects the properties of chemical substances, in other words a link between what’s happening on the atomic and molecular scale and the world we can see.