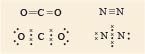1.3 Multiple bonds
So far, any two atoms in the Lewis structures you have looked at have been held together by just one electron pair bond.
Logically this is called a single bond.
But sometimes two or even three shared pairs may be involved, two examples are shown in Figure 1, however the name of the game is still the attainment of eight outer electrons.
In carbon dioxide, the two electron pairs between each oxygen atom and the central carbon atom represent each carbon-oxygen double bond.
Turning now to the Lewis structure of the nitrogen molecule, the three shared pairs in the nitrogen molecule are equivalent to a triple bond.
As an aside, it is worth adding that nitrogen, N2, is chemically very unreactive, in fact almost as inert as the noble gases. This stems from the great strength of this triple bond. Indeed, gaseous nitrogen finds applications where inert atmospheres are required, including manufacture of air-sensitive chemicals, and in the liquid form when extreme cold is essential (Figure 2), such as the preservation of blood and semen, and rapid freezing of food.
You can see some demonstrations involving liquid nitrogen in the following video.