1.4 Linking Lewis structures with valency
The Lewis structures that you have look at so far, tell you why carbon, hydrogen, nitrogen, oxygen and chlorine have the valencies they have.
When atoms are forming covalent bonds, the valency is the number of shared electron-pair bonds that the atom must form if it is to attain the electron arrangement of a noble gas.
Thus hydrogen and oxygen form water, H2O, rather than HO or HO2, because H2O enables both elements to attain the structure of a noble gas.
Oxygen gas and sulfur dichloride contain the molecules O2 and SCl2 respectively. Draw a Lewis structure for each molecule. Which noble gas structure is achieved by each atom in the molecules?
With six outer electrons each, the two oxygen atoms in the O2 molecule must form a double bond (two shared electron pairs, structure 4.16) with each other if each is to attain a noble gas configuration. SCl2 contains single bonds (structure 4.17 ).
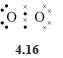
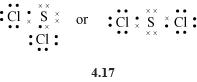
In these Lewis structures, oxygen attains the neon configuration, and sulfur and chlorine the argon configuration. When drawing Lewis structures, shape is unimportant; the important point is to show the correct number of bonds (shared electron pairs) formed by each atom. As either form of structure 4.17 does the job, both are acceptable.
